当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The C5-substituent effects on the formic acid-assisted tautomerization of protonated cytosine: A lower isomerization barrier and potential biological importance
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-05-21 , DOI: 10.1002/poc.4220 Lingxia Jin 1 , Mengdan Lv 1 , Shengnan Shi 1 , Jiufu Lu 1 , Qin Wang 1 , Xiaohu Yu 1 , Wendeng Huang 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-05-21 , DOI: 10.1002/poc.4220 Lingxia Jin 1 , Mengdan Lv 1 , Shengnan Shi 1 , Jiufu Lu 1 , Qin Wang 1 , Xiaohu Yu 1 , Wendeng Huang 1
Affiliation
|
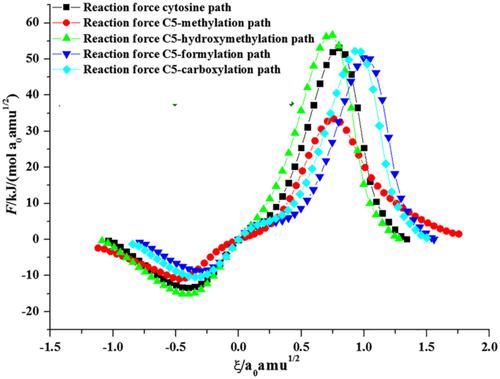
The continuous oxidative products of 5-methylcytosine are called the “three new DNA bases.” When it comes to 5-formylcytosine and 5-carboxylcytosine, the electron densities at N3 sites of both bases tend to be decreased due to the presence of the electron withdrawing groups of CHO and COOH. The vital steps for mutations of DNA are tautomerism in nucleotide bases. Although there are great deal of studies on the protonated new DNA bases in photophysical and photochemical reactivity, the relationship of pKa at N3 position with the intermolecular tautomerization barrier is seldom reported. The C5 atom of cytosine is substituted by the CH3, HOCH2, CHO, and COOH, and their isomerization barriers in the presence of HCOOH have nearly linear relationship with the pKa at N3 positions of these bases. The solvent water affects the activation free energies of these paths, and yet their isomerization mechanisms are still more favorable in aqueous solution. Meantime, the rate constants could be calculated by the conventional transition state theory with Wigner's tunneling correction. The corrected rate constant for these paths is very consistent with uncorrected results. Finally, the product and reaction complexes are in a fast tautomerism equilibrium, which is a synchronous double proton transfer mechanism. The product complexes may further dissociated into the monomers. These researches may give a chemical basis for differentiating 5-fCyt and 5-caCyt from Cyt, 5-MeCyt, and 5-HCyt in the protein-DNA interactions which might be used for selective recognition.
中文翻译:

C5 取代基对甲酸辅助质子化胞嘧啶互变异构化的影响:较低的异构化屏障和潜在的生物学重要性
5-甲基胞嘧啶的连续氧化产物被称为“三个新的DNA碱基”。当涉及5-甲酰基胞嘧啶和5-羧基胞嘧啶时,由于CHO和COOH的吸电子基团的存在,两个碱基的N3位点的电子密度趋于降低。DNA 突变的关键步骤是核苷酸碱基的互变异构。尽管在光物理和光化学反应性方面对质子化的新 DNA 碱基进行了大量研究,但很少报道 N3 位置的 pKa 与分子间互变异构屏障的关系。胞嘧啶的 C5 原子被 CH 3、 HOCH 2、 CHO 和COOH 及其在 HCOOH 存在下的异构化势垒与这些碱基的 N3 位置的 pKa 几乎呈线性关系。溶剂水影响这些途径的活化自由能,但它们的异构化机制在水溶液中仍然更有利。同时,速率常数可以通过传统的过渡态理论和 Wigner 隧道校正来计算。这些路径的校正速率常数与未校正结果非常一致。最后,产物和反应配合物处于快速互变异构平衡,这是一种同步双质子转移机制。产物复合物可进一步离解成单体。这些研究可能为区分 5-fCyt 和 5-caCyt 与 Cyt、5-MeCyt、
更新日期:2021-05-21
中文翻译:

C5 取代基对甲酸辅助质子化胞嘧啶互变异构化的影响:较低的异构化屏障和潜在的生物学重要性
5-甲基胞嘧啶的连续氧化产物被称为“三个新的DNA碱基”。当涉及5-甲酰基胞嘧啶和5-羧基胞嘧啶时,由于CHO和COOH的吸电子基团的存在,两个碱基的N3位点的电子密度趋于降低。DNA 突变的关键步骤是核苷酸碱基的互变异构。尽管在光物理和光化学反应性方面对质子化的新 DNA 碱基进行了大量研究,但很少报道 N3 位置的 pKa 与分子间互变异构屏障的关系。胞嘧啶的 C5 原子被 CH 3、 HOCH 2、 CHO 和COOH 及其在 HCOOH 存在下的异构化势垒与这些碱基的 N3 位置的 pKa 几乎呈线性关系。溶剂水影响这些途径的活化自由能,但它们的异构化机制在水溶液中仍然更有利。同时,速率常数可以通过传统的过渡态理论和 Wigner 隧道校正来计算。这些路径的校正速率常数与未校正结果非常一致。最后,产物和反应配合物处于快速互变异构平衡,这是一种同步双质子转移机制。产物复合物可进一步离解成单体。这些研究可能为区分 5-fCyt 和 5-caCyt 与 Cyt、5-MeCyt、































 京公网安备 11010802027423号
京公网安备 11010802027423号