当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-(Pyridin-2-yl)isopropyl (PIP) Amine: An Enabling Directing Group for Divergent and Asymmetric Functionalization of Unactivated Methylene C(sp3)–H Bonds
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-05-21 , DOI: 10.1021/acs.accounts.1c00168 Qi Zhang 1 , Bing-Feng Shi 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-05-21 , DOI: 10.1021/acs.accounts.1c00168 Qi Zhang 1 , Bing-Feng Shi 1
Affiliation
|
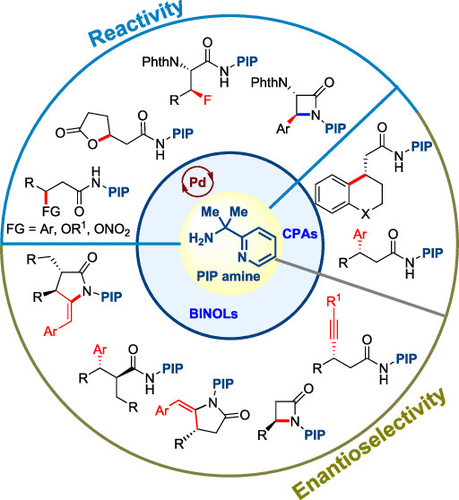
Directing group (DG) assistance provides a good solution to the problems of reactivity and selectivity, two of the fundamental challenges in C(sp3)–H activation. However, the activation of unbiased methylene C(sp3)–H bonds remains challenging due to the high heterolytic bond dissociation energy and substantial steric hindrance. Two main strategies have been developed thus far, that is, use of a strongly coordinating bidentate DG pioneered by Daugulis and use of a weakly coordinating monodentate DG accelerated by pyridine-type ligands, as disclosed by Yu. The seminal work by Daugulis sparked significant interest in the application of the monoanionic bidentate auxiliary in aliphatic C–H activation reactions. Our research has focused on enabling the divergent functionalization and enantiotopic differentiation of unactivated methylene C–H bonds. Inspired by the structure of bidentate 8-aminoquinoline and the accelerating effect of the gem-dimethyl moiety in cyclometalations, we developed a strongly coordinating bidentate 2-(pyridine-yl)isopropyl (PIP) amine DG consisting of a pyridyl group, a gem-dimethyl moiety, and an amino group, which enabled the divergent functionalization of unactivated β-methylene C(sp3)–H bonds to forge C–O, C–N, C–C, and C–F bonds with palladium catalysts. The exclusive β-selectivity was ascribed to the preferential formation of kinetically favored [5,5]-bicyclic palladacycle intermediates. DFT calculations revealed that the well-designed gem-dimethyl group was responsible for the lowered energy and compressed bite angle of the key transition state related to C–H cleavage.
中文翻译:

2-(吡啶-2-基)异丙基(PIP)胺:未活化亚甲基C(sp3)-H键发散和不对称官能化的引导基团
定向基团 (DG) 辅助为反应性和选择性问题提供了一个很好的解决方案,这是 C(sp 3 )–H 活化中的两个基本挑战。然而,无偏亚甲基 C(sp 3)–H 键由于高异裂键解离能和大量空间位阻仍然具有挑战性。到目前为止,已经开发了两种主要策略,即使用由 Daugulis 开创的强配位双齿 DG 和使用由吡啶型配体加速的弱配位单齿 DG,如 Yu 所公开的。Daugulis 的开创性工作激发了人们对单阴离子双齿助剂在脂肪族 C-H 活化反应中的应用的浓厚兴趣。我们的研究重点是使未活化的亚甲基 C-H 键的不同功能化和对映体分化成为可能。灵感来自双齿 8-氨基喹啉的结构和宝石的加速作用环金属化中的二甲基部分,我们开发了一种强配位的双齿 2-(吡啶-基)异丙基(PIP)胺 DG,由一个吡啶基、一个gem-二甲基部分和一个氨基组成,这使得未活化的 β 能够发散功能化-亚甲基 C(sp 3 )–H 键与钯催化剂形成 C–O、C–N、C–C 和 C–F 键。独特的β-选择性归因于优先形成动力学有利的[5,5]-双环钯环中间体。DFT 计算表明,精心设计的gem-二甲基基团导致与 C-H 解理相关的关键过渡态的能量降低和咬合角压缩。
更新日期:2021-06-15
中文翻译:

2-(吡啶-2-基)异丙基(PIP)胺:未活化亚甲基C(sp3)-H键发散和不对称官能化的引导基团
定向基团 (DG) 辅助为反应性和选择性问题提供了一个很好的解决方案,这是 C(sp 3 )–H 活化中的两个基本挑战。然而,无偏亚甲基 C(sp 3)–H 键由于高异裂键解离能和大量空间位阻仍然具有挑战性。到目前为止,已经开发了两种主要策略,即使用由 Daugulis 开创的强配位双齿 DG 和使用由吡啶型配体加速的弱配位单齿 DG,如 Yu 所公开的。Daugulis 的开创性工作激发了人们对单阴离子双齿助剂在脂肪族 C-H 活化反应中的应用的浓厚兴趣。我们的研究重点是使未活化的亚甲基 C-H 键的不同功能化和对映体分化成为可能。灵感来自双齿 8-氨基喹啉的结构和宝石的加速作用环金属化中的二甲基部分,我们开发了一种强配位的双齿 2-(吡啶-基)异丙基(PIP)胺 DG,由一个吡啶基、一个gem-二甲基部分和一个氨基组成,这使得未活化的 β 能够发散功能化-亚甲基 C(sp 3 )–H 键与钯催化剂形成 C–O、C–N、C–C 和 C–F 键。独特的β-选择性归因于优先形成动力学有利的[5,5]-双环钯环中间体。DFT 计算表明,精心设计的gem-二甲基基团导致与 C-H 解理相关的关键过渡态的能量降低和咬合角压缩。

































 京公网安备 11010802027423号
京公网安备 11010802027423号