Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-05-21 , DOI: 10.1016/j.seppur.2021.118989 Li Cui , Shasha Li , Jin Kang , Caixia Yin , Yanxia Guo , Hongyan He , Fangqin Cheng
|
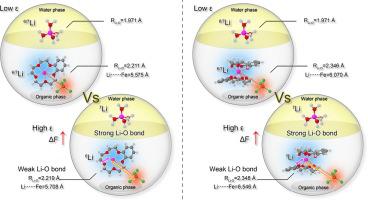
Effective separation of lithium isotopes (6Li and 7Li) is of great importance for the development of nuclear energy. Crown ethers (CEs) are widely used and reported separation systems, but the distribution coefficient of Li (DLi) is relatively low by CEs (DLi, 10−2–10−5) and mainly depends on the anion type of Li salt. Herein, a novel and facile ion-pair strategy by introducing tetrachloridoferrate ([FeCl4]−) as counter anion is presented, which helps to overcome the Hofmeister bias and facilitates the efficient transfer of Li+ from extremely hydrophilic chlorides. An exceptionally high DLi of 54 is achieved for benzo-15-crown-5 (B15C5), surpassing those of solvent extraction from solely Li salt aqueous solution. This strategy also demonstrates good separation performance of 6Li/7Li. The maximum 6Li/7Li separation factors () of 1.038 and 1.049 are obtained for B15C5 and benzo-12-crown-4 (B12C4) in dichloroethane at 273 K. The highly efficient phase transfer of Li+ can be attributed to the formation of stable [Li(B15C5)(H2O)][FeCl4] or [Li(B12C4)2][FeCl4] ion pairs. Theoretical studies based on density functional theory (DFT) calculations suggest that the coordination and electrostatic environment of Li+ plays an essential role in the separation of 6Li/7Li. Weakening the coordination and electrostatic interactions associated with Li+ can facilitate the separation of 6Li/7Li. Additionally, B15C5 can be easily recycled by using ultra-pure water as the stripping solution. This work provides an industrially promising system for 6Li/7Li separation.
中文翻译:

一种使用冠醚有效分离锂同位素的新型离子对策略
锂同位素(6 Li和7 Li)的有效分离对核能的发展具有重要意义。冠醚 (CEs) 是广泛使用和报道的分离系统,但CEs ( D Li , 10 - 2 –10 -5 )的 Li ( D Li )分配系数相对较低,主要取决于锂盐的阴离子类型. 在此,通过引入四氯化铁([FeCl 4 ] -)作为抗衡阴离子,提出了一种新颖且简便的离子对策略,这有助于克服 Hofmeister 偏差并促进 Li +的有效转移。来自极亲水的氯化物。苯并 15-冠 5 (B15C5) 的D Li达到了 54的异常高,超过了仅从锂盐水溶液中溶剂萃取的结果。该策略还展示了6 Li/ 7 Li 的良好分离性能。最大6 Li/ 7 Li 分离因子 (B15C5 和 benzo-12-crown-4 (B12C4) 在 273 K 的二氯乙烷中获得 1.038 和 1.049 的 )。 Li + 的高效相转移可归因于稳定 [Li(B15C5)(H 2 O)][FeCl 4 ] 或 [Li(B12C4) 2 ][FeCl 4 ] 离子对。基于密度泛函理论 (DFT) 计算的理论研究表明,Li +的配位和静电环境在6 Li/ 7 Li的分离中起着至关重要的作用。减弱与 Li +相关的配位和静电相互作用可以促进6 Li/ 7的分离李。此外,B15C5 可以通过使用超纯水作为汽提液轻松回收。这项工作为6 Li/ 7 Li 分离提供了一个具有工业前景的系统。






























 京公网安备 11010802027423号
京公网安备 11010802027423号