当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tale of the Breslow intermediate, a central player in N-heterocyclic carbene organocatalysis: then and now
Chemical Science ( IF 7.6 ) Pub Date : 2021-5-11 , DOI: 10.1039/d1sc01910d Monika Pareek 1 , Yernaidu Reddi 1 , Raghavan B Sunoj 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-5-11 , DOI: 10.1039/d1sc01910d Monika Pareek 1 , Yernaidu Reddi 1 , Raghavan B Sunoj 1
Affiliation
|
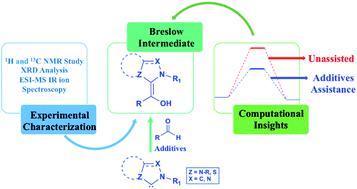
N-Heterocyclic carbenes (NHCs) belong to the popular family of organocatalysts used in a wide range of reactions, including that for the synthesis of complex natural products and biologically active compounds. In their organocatalytic manifestation, NHCs are known to impart umpolung reactivity to aldehydes and ketones, which are then exploited in the generation of homoenolate, acyl anion, and enolate equivalents suitable for a plethora of reactions such as annulation, benzoin, Stetter, Claisen rearrangement, cycloaddition, and C–C and C–H bond functionalization reactions and so on. A common thread that runs through these NHC catalyzed reactions is the proposed involvement of an enaminol, also known as the Breslow intermediate, formed by the nucleophilic addition of an NHC to a carbonyl group of a suitable electrophile. In the emerging years of NHC catalysis, enaminol remained elusive and was largely considered a putative intermediate owing to the difficulties encountered in its isolation and characterization. However, in the last decade, synergistic efforts utilizing an array of computational and experimental techniques have helped in gaining important insights into the formation and characterization of Breslow intermediates. Computational studies have suggested that a direct 1,2-proton transfer within the initial zwitterionic intermediate, generated by the action of an NHC on the carbonyl carbon, is energetically prohibitive and hence the participation of other species capable of promoting an assisted proton transfer is more likely. The proton transfer assisted by additives (such as acids, bases, other species, or even a solvent) was found to ease the kinetics of formation of Breslow intermediates. These important details on the formation, in situ detection, isolation, and characterization of the Breslow intermediate are scattered over a series of reports spanning well over a decade, and we intend to consolidate them in this review and provide a critical assessment of these developments. Given the central role of the Breslow intermediate in organocatalytic reactions, this treatise is expected to serve as a valuable source of knowledge on the same.
中文翻译:

Breslow 中间体的故事,N-杂环卡宾有机催化的核心参与者:过去和现在
N-杂环卡宾 (NHC) 属于流行的有机催化剂家族,用于广泛的反应,包括用于合成复杂的天然产物和生物活性化合物的反应。在其有机催化表现中,NHC 已知可赋予醛和酮的反极性反应性,然后将其用于生成高烯醇盐、酰基阴离子和烯醇盐等价物,适用于多种反应,例如环化、安息香、Stetter、克莱森重排、环加成反应、C-C、C-H键功能化反应等。贯穿这些 NHC 催化反应的一个共同点是烯醇(也称为 Breslow 中间体)的参与,它是通过 NHC 与合适的亲电子试剂的羰基进行亲核加成而形成的。在 NHC 催化的新兴年代,烯醇仍然难以捉摸,并且由于在分离和表征方面遇到困难,在很大程度上被认为是假定的中间体。然而,在过去的十年中,利用一系列计算和实验技术的协同努力有助于获得对 Breslow 中间体的形成和表征的重要见解。计算研究表明,由 NHC 对羰基碳的作用产生的初始两性离子中间体中的直接 1,2-质子转移在能量上是被禁止的,因此能够促进辅助质子转移的其他物种的参与更加重要可能。人们发现,添加剂(例如酸、碱、其他物质,甚至溶剂)辅助的质子转移可以缓解 Breslow 中间体形成的动力学。这些关于 Breslow 中间体的形成、原位检测、分离和表征的重要细节分散在十多年来的一系列报告中,我们打算在本次综述中对它们进行整合,并对这些进展进行批判性评估。鉴于布雷斯洛中间体在有机催化反应中的核心作用,本文有望成为有关该领域的宝贵知识来源。
更新日期:2021-05-22
中文翻译:

Breslow 中间体的故事,N-杂环卡宾有机催化的核心参与者:过去和现在
N-杂环卡宾 (NHC) 属于流行的有机催化剂家族,用于广泛的反应,包括用于合成复杂的天然产物和生物活性化合物的反应。在其有机催化表现中,NHC 已知可赋予醛和酮的反极性反应性,然后将其用于生成高烯醇盐、酰基阴离子和烯醇盐等价物,适用于多种反应,例如环化、安息香、Stetter、克莱森重排、环加成反应、C-C、C-H键功能化反应等。贯穿这些 NHC 催化反应的一个共同点是烯醇(也称为 Breslow 中间体)的参与,它是通过 NHC 与合适的亲电子试剂的羰基进行亲核加成而形成的。在 NHC 催化的新兴年代,烯醇仍然难以捉摸,并且由于在分离和表征方面遇到困难,在很大程度上被认为是假定的中间体。然而,在过去的十年中,利用一系列计算和实验技术的协同努力有助于获得对 Breslow 中间体的形成和表征的重要见解。计算研究表明,由 NHC 对羰基碳的作用产生的初始两性离子中间体中的直接 1,2-质子转移在能量上是被禁止的,因此能够促进辅助质子转移的其他物种的参与更加重要可能。人们发现,添加剂(例如酸、碱、其他物质,甚至溶剂)辅助的质子转移可以缓解 Breslow 中间体形成的动力学。这些关于 Breslow 中间体的形成、原位检测、分离和表征的重要细节分散在十多年来的一系列报告中,我们打算在本次综述中对它们进行整合,并对这些进展进行批判性评估。鉴于布雷斯洛中间体在有机催化反应中的核心作用,本文有望成为有关该领域的宝贵知识来源。

































 京公网安备 11010802027423号
京公网安备 11010802027423号