当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insights into the Actinide–Silicon Bonding Nature and Stability of a Series of Actinide Complexes with Different Oxidation States
Organometallics ( IF 2.5 ) Pub Date : 2021-05-21 , DOI: 10.1021/acs.organomet.1c00196 Ai-Lin Li 1, 2 , Nai-Xin Zhang 1, 2 , Qun-Yan Wu 2 , Cong-Zhi Wang 2 , Jian-Hui Lan 2 , Chang-Ming Nie 1 , Zhi-Fang Chai 2, 3 , Wei-Qun Shi 2
Organometallics ( IF 2.5 ) Pub Date : 2021-05-21 , DOI: 10.1021/acs.organomet.1c00196 Ai-Lin Li 1, 2 , Nai-Xin Zhang 1, 2 , Qun-Yan Wu 2 , Cong-Zhi Wang 2 , Jian-Hui Lan 2 , Chang-Ming Nie 1 , Zhi-Fang Chai 2, 3 , Wei-Qun Shi 2
Affiliation
|
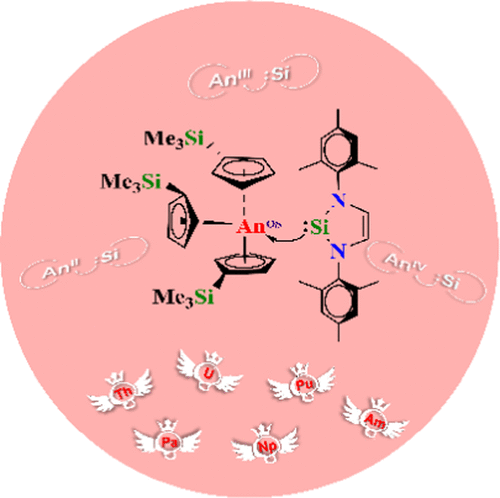
The electronic structures of a series of complexes (CpSiMe3)3AnSi(NCHMes)2 ([An–Si], An = Th–Am) with different oxidation states (OS = II, III, and IV) of actinides were investigated using the relativistic density functional theory to explore the actinide–silicon bonding, which was evaluated based on the analyses of quantum theory of atoms in molecules (QTAIM) and electron localization function (ELF). The An–Si bond length variation for a series of [An–Si] with different oxidation states may be attributed to the synergistic effect of the steric hindrance and the ability of the actinide atom to accept electrons. The value of An–Si Mayer bond order (MBO) decreases across the actinide series with the same oxidation state. The values of An–Si MBO for the [AnII–Si]− (An = Th–Pu) are the largest in general among the complexes with three actinide oxidation states, except that for the Am–Si MBO of [AmIV–Si]+ is the largest because of more covalent Am–Si bond in the [AmIV–Si]+ complex. The An–Si bonds are highly polarized due to lone pair electrons located on the Si 3s orbital. Moreover, the An–Si bonds possess donor–acceptor interactions according to the analyses of QTAIM and ELF. In addition, the binding energies suggest that the tetravalent complexes [AnIV–Si]+ are thermodynamically accessible, of which [UIV–Si]+ and [PuIV–Si]+ are the most preferable. The electron affinity analysis suggests that the reduction reaction of [AnIII–Si] → [AnII–Si]− should become increasingly facile across the actinide series from Th to Am. This work expands the knowledge on the An–Si bonding, especially for the transuranium–silicon bonding, and guides synthesis of the actinide silicon complexes with different oxidation states.
中文翻译:

一系列不同氧化态锕系配合物的锕系-硅键合性质和稳定性的理论见解
使用锕系元素的不同氧化态 (OS = II、III 和 IV) 研究了一系列配合物 (CpSiMe 3 ) 3 AnSi(NCHMes) 2 ([An-Si], An = Th-Am)的电子结构相对论密度泛函理论探索锕系 - 硅键合,这是基于分子中原子的量子理论(QTAIM)和电子局域化函数(ELF)的分析来评估的。一系列具有不同氧化态的 [An-Si] 的 An-Si 键长变化可能归因于空间位阻的协同效应和锕系原子接受电子的能力。An-Si Mayer 键序 (MBO) 的值在具有相同氧化态的锕系系列中降低。An-Si MBO 的值 [AnII -Si] - (An = Th-Pu) 在具有三种锕系元素氧化态的配合物中一般是最大的,除了 [Am IV -Si] +的 Am-Si MBO是最大的,因为更多的共价 Am [Am IV -Si] +络合物中的 -Si 键。由于位于 Si 3s 轨道上的孤对电子,An-Si 键高度极化。此外,根据 QTAIM 和 ELF 的分析,An-Si 键具有供体-受体相互作用。此外,结合能表明四价配合物 [An IV -Si] +是热力学可接近的,其中 [U IV -Si] +和 [Pu IV-Si] +是最优选的。电子亲和性分析表明 [An III -Si] → [An II -Si] -的还原反应应该在从 Th 到 Am 的锕系系列中变得越来越容易。这项工作扩展了关于 An-Si 键合的知识,尤其是超铀-硅键合,并指导了具有不同氧化态的锕系硅配合物的合成。
更新日期:2021-06-14
中文翻译:

一系列不同氧化态锕系配合物的锕系-硅键合性质和稳定性的理论见解
使用锕系元素的不同氧化态 (OS = II、III 和 IV) 研究了一系列配合物 (CpSiMe 3 ) 3 AnSi(NCHMes) 2 ([An-Si], An = Th-Am)的电子结构相对论密度泛函理论探索锕系 - 硅键合,这是基于分子中原子的量子理论(QTAIM)和电子局域化函数(ELF)的分析来评估的。一系列具有不同氧化态的 [An-Si] 的 An-Si 键长变化可能归因于空间位阻的协同效应和锕系原子接受电子的能力。An-Si Mayer 键序 (MBO) 的值在具有相同氧化态的锕系系列中降低。An-Si MBO 的值 [AnII -Si] - (An = Th-Pu) 在具有三种锕系元素氧化态的配合物中一般是最大的,除了 [Am IV -Si] +的 Am-Si MBO是最大的,因为更多的共价 Am [Am IV -Si] +络合物中的 -Si 键。由于位于 Si 3s 轨道上的孤对电子,An-Si 键高度极化。此外,根据 QTAIM 和 ELF 的分析,An-Si 键具有供体-受体相互作用。此外,结合能表明四价配合物 [An IV -Si] +是热力学可接近的,其中 [U IV -Si] +和 [Pu IV-Si] +是最优选的。电子亲和性分析表明 [An III -Si] → [An II -Si] -的还原反应应该在从 Th 到 Am 的锕系系列中变得越来越容易。这项工作扩展了关于 An-Si 键合的知识,尤其是超铀-硅键合,并指导了具有不同氧化态的锕系硅配合物的合成。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号