当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Trans-4a,12b/3,4-Dihydrodibenzo[f,h]quinolin-2(1H)-Ones and Dibenzo[f,h]quinolin-2(1H)-Ones via Irradiation of 6-Biphenylpyridine-2(1H)-Ones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-05-21 , DOI: 10.1002/adsc.202100509 jingzhi sui 1 , yun he 1 , Tao Wang 2 , Yong Liang 3 , Zunting Zhang 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-05-21 , DOI: 10.1002/adsc.202100509 jingzhi sui 1 , yun he 1 , Tao Wang 2 , Yong Liang 3 , Zunting Zhang 2
Affiliation
|
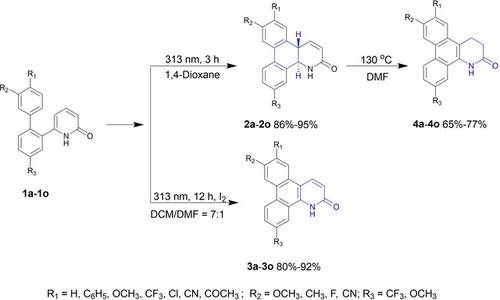
The synthesis of trans-4a,12b-dihydrodibenzo[f,h]quinolin-2(1H)-ones (2), dibenzo[f,h]quinolin-2(1H)-ones (3) and 3,4-dihydrodibenzo[f,h]quinolin-2(1H)-ones (4) via photo-induced annulation of 6-([1,1′-biphenyl]-2-yl)pyridine-2(1H)-ones (1) under irradiation of a 313 nm UV light was described. Compounds 2 were obtained in 82–95% yields when the irradiation time was 3 h. Products 3 were obtained by irradiating 1 for 12 h in the presence of iodine. Heating the solutions of 2 in DMF at 130 °C for 24 h gave compounds 4 via a double 1,3-H shift. The demonstrated protocols showed the diversity of photo-induced cyclization of compounds 1.
中文翻译:

Trans-4a,12b/3,4-Dihydrodibenzo[f,h]quinolin-2(1H)-Ones 和 Dibenzo[f,h]quinolin-2(1H)-Ones 通过 6-Biphenylpyridine-2( 1H)-个
的合成反式-4一个,12 b二氢二苯[ ˚F,ħ ]喹啉-2(1 H ^) -酮(2) ,二苯并[ ˚F,ħ ]喹啉-2(1 H ^) -酮(3)和3 ,4-dihydrodibenzo[ f , h ]quinolin-2(1 H )-ones ( 4) 通过光致环化 6-([1,1'-biphenyl]-2-yl)pyridine-2(1 H ) -ones ( 1)在 313 nm 紫外光照射下进行了描述。化合物2当辐照时间为 3 小时时,产率为 82-95%。通过在碘存在下照射1 12 小时获得产物3。将2在 DMF 中的溶液在 130 °C 下加热24 小时,通过双 1,3-H 位移得到化合物4。演示的协议显示了化合物1的光诱导环化的多样性。
更新日期:2021-07-20
中文翻译:

Trans-4a,12b/3,4-Dihydrodibenzo[f,h]quinolin-2(1H)-Ones 和 Dibenzo[f,h]quinolin-2(1H)-Ones 通过 6-Biphenylpyridine-2( 1H)-个
的合成反式-4一个,12 b二氢二苯[ ˚F,ħ ]喹啉-2(1 H ^) -酮(2) ,二苯并[ ˚F,ħ ]喹啉-2(1 H ^) -酮(3)和3 ,4-dihydrodibenzo[ f , h ]quinolin-2(1 H )-ones ( 4) 通过光致环化 6-([1,1'-biphenyl]-2-yl)pyridine-2(1 H ) -ones ( 1)在 313 nm 紫外光照射下进行了描述。化合物2当辐照时间为 3 小时时,产率为 82-95%。通过在碘存在下照射1 12 小时获得产物3。将2在 DMF 中的溶液在 130 °C 下加热24 小时,通过双 1,3-H 位移得到化合物4。演示的协议显示了化合物1的光诱导环化的多样性。































 京公网安备 11010802027423号
京公网安备 11010802027423号