当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Construction of Pyrimidine-Fused Diazepinone Derivatives Bearing a Tertiary Stereogenic Center Enabled by Iridium-Catalysed Intramolecular Allylic Substitution
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-05-21 , DOI: 10.1002/adsc.202100444 Xiaoding Jiang 1 , Bendu Pan 1 , Xu Qian 1 , Hao Liang 1 , Yaqi Zhang 2 , Bin Chen 3 , Xiaobo He 2 , Hoi-Shan Chan 4 , Albert S. C. Chan 2 , Liqin Qiu 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-05-21 , DOI: 10.1002/adsc.202100444 Xiaoding Jiang 1 , Bendu Pan 1 , Xu Qian 1 , Hao Liang 1 , Yaqi Zhang 2 , Bin Chen 3 , Xiaobo He 2 , Hoi-Shan Chan 4 , Albert S. C. Chan 2 , Liqin Qiu 2
Affiliation
|
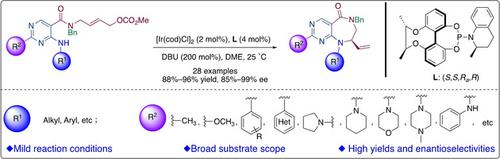
The iridium-catalysed enantioselective intramolecular allylic substitution of pyrimidine-tethered allylic carbonates was developed. A wide range of chiral pyrimidine-fused diazepinone derivatives were successfully constructed in 88–96% yields with 85–99% ees. This work further highlights the power of chiral-bridged biphenyl phosphoramidites in asymmetric synthesis.
中文翻译:

通过铱催化的分子内烯丙基取代,具有三级立体中心的嘧啶稠合二氮杂酮衍生物的对映选择性构建
开发了铱催化的对映选择性分子内烯丙基取代嘧啶系烯丙基碳酸酯。以 88-96% 的产率和 85-99% 的 ees 成功构建了多种手性嘧啶稠合的二氮杂酮衍生物。这项工作进一步突出了手性桥连联苯亚磷酰胺在不对称合成中的作用。
更新日期:2021-07-04
中文翻译:

通过铱催化的分子内烯丙基取代,具有三级立体中心的嘧啶稠合二氮杂酮衍生物的对映选择性构建
开发了铱催化的对映选择性分子内烯丙基取代嘧啶系烯丙基碳酸酯。以 88-96% 的产率和 85-99% 的 ees 成功构建了多种手性嘧啶稠合的二氮杂酮衍生物。这项工作进一步突出了手性桥连联苯亚磷酰胺在不对称合成中的作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号