当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Harnessing In Situ Radical Oxygenation: Copper-Catalyzed Interrupted Azirine–Alkyne Ring-Expansion Reaction for the Synthesis of Pyrrolones
Organic Letters ( IF 4.9 ) Pub Date : 2021-05-19 , DOI: 10.1021/acs.orglett.1c01162 Chandragiri Sujatha 1 , Madhu Nallagangula 1 , Kayambu Namitharan 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2021-05-19 , DOI: 10.1021/acs.orglett.1c01162 Chandragiri Sujatha 1 , Madhu Nallagangula 1 , Kayambu Namitharan 1, 2
Affiliation
|
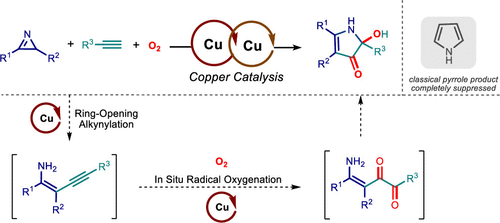
Here we report a novel interrupted azirine–alkyne ring-expansion reaction with molecular oxygen for the direct synthesis of highly functionalized pyrrolones enabled by copper catalysis. Mechanistic investigations indicate that the present three-component reaction proceeds via two copper-catalyzed sequential reactions, an azirine-ring-opening alkynylation and an amine-directed radical oxygenation, leading to the formation of interesting pyrrolone structures under mild conditions.
中文翻译:

利用原位自由基氧化:用于合成吡咯酮的铜催化间断氮-炔环膨胀反应
在这里,我们报告了一种新型的中断式氮丙啶-炔烃扩环反应,用于通过铜催化直接合成高度官能化的吡咯酮。机理研究表明,目前的三组分反应通过两个铜催化的连续反应、氮杂环开环炔基化和胺导向的自由基氧化进行,导致在温和条件下形成有趣的吡咯酮结构。
更新日期:2021-06-04
中文翻译:

利用原位自由基氧化:用于合成吡咯酮的铜催化间断氮-炔环膨胀反应
在这里,我们报告了一种新型的中断式氮丙啶-炔烃扩环反应,用于通过铜催化直接合成高度官能化的吡咯酮。机理研究表明,目前的三组分反应通过两个铜催化的连续反应、氮杂环开环炔基化和胺导向的自由基氧化进行,导致在温和条件下形成有趣的吡咯酮结构。































 京公网安备 11010802027423号
京公网安备 11010802027423号