当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lattice oxygen self-spillover on reducible oxide supported metal cluster: the water–gas shift reaction on Cu/CeO2 catalyst
Chemical Science ( IF 7.6 ) Pub Date : 2021-5-12 , DOI: 10.1039/d1sc01201k Ya-Qiong Su 1, 2, 3 , Guang-Jie Xia 1 , Yanyang Qin 2 , Shujiang Ding 2 , Yang-Gang Wang 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-5-12 , DOI: 10.1039/d1sc01201k Ya-Qiong Su 1, 2, 3 , Guang-Jie Xia 1 , Yanyang Qin 2 , Shujiang Ding 2 , Yang-Gang Wang 1
Affiliation
|
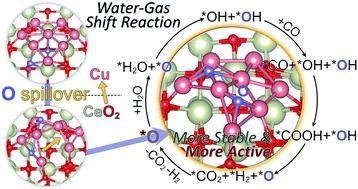
In this work we have tackled one of the most challenging problems in nanocatalysis namely understanding the role of reducible oxide supports in metal catalyzed reactions. As a prototypical example, the very well-studied water gas shift reaction catalyzed by CeO2 supported Cu nanoclusters is chosen to probe how the reducible oxide support modifies the catalyst structures, catalytically active sites and even the reaction mechanisms. By employing density functional theory calculations in conjunction with a genetic algorithm and ab initio molecular dynamics simulations, we have identified an unprecedented spillover of the surface lattice oxygen from the ceria support to the Cu cluster, which is rarely considered previously but may widely exist in oxide supported metal catalysts under realistic conditions. The oxygen spillover causes a highly energetic preference of the monolayered configuration of the supported Cu nanocluster, compared to multilayered configurations. Due to the strong metal–oxide interaction, after the O spillover the monolayered cluster is highly oxidized by transferring electrons to the Ce 4f orbitals. The water–gas-shift reaction is further found to more favorably take place on the supported copper monolayer than the copper-ceria periphery, where the on-site oxygen and the adjacent oxidized Cu sites account for the catalytically active sites, synergistically facilitating the water dissociation and the carboxyl formation. The present work provides mechanistic insights into the strong metal–support interaction and its role in catalytic reactions, which may pave a way towards the rational design of metal–oxide catalysts with promising stability, dispersion and catalytic activity.
中文翻译:

可还原氧化物负载金属簇上的晶格氧自溢出:Cu/CeO2 催化剂上的水煤气变换反应
在这项工作中,我们解决了纳米催化中最具挑战性的问题之一,即了解可还原氧化物载体在金属催化反应中的作用。作为一个典型的例子,选择了经过充分研究的由CeO 2负载的Cu纳米团簇催化的水煤气变换反应来探讨可还原氧化物载体如何改变催化剂结构、催化活性位点甚至反应机制。通过采用密度泛函理论计算结合遗传算法和从头算分子动力学模拟,我们发现了表面晶格氧从二氧化铈载体到铜簇的前所未有的溢出,这种溢出以前很少被考虑,但可能广泛存在于氧化物中现实条件下的负载金属催化剂。与多层结构相比,氧气溢出导致负载铜纳米团簇的单层结构具有高能量偏好。由于强烈的金属-氧化物相互作用,在 O 溢出后,单层团簇通过将电子转移到 Ce 4f 轨道而被高度氧化。进一步发现,水煤气变换反应在负载的铜单层上比在铜-二氧化铈外围更有利地发生,其中现场氧和相邻的氧化铜位点占据催化活性位点,协同促进水的生成。解离和羧基形成。目前的工作为强金属-载体相互作用及其在催化反应中的作用提供了机理见解,这可能为合理设计具有良好稳定性、分散性和催化活性的金属氧化物催化剂铺平道路。
更新日期:2021-05-20
中文翻译:

可还原氧化物负载金属簇上的晶格氧自溢出:Cu/CeO2 催化剂上的水煤气变换反应
在这项工作中,我们解决了纳米催化中最具挑战性的问题之一,即了解可还原氧化物载体在金属催化反应中的作用。作为一个典型的例子,选择了经过充分研究的由CeO 2负载的Cu纳米团簇催化的水煤气变换反应来探讨可还原氧化物载体如何改变催化剂结构、催化活性位点甚至反应机制。通过采用密度泛函理论计算结合遗传算法和从头算分子动力学模拟,我们发现了表面晶格氧从二氧化铈载体到铜簇的前所未有的溢出,这种溢出以前很少被考虑,但可能广泛存在于氧化物中现实条件下的负载金属催化剂。与多层结构相比,氧气溢出导致负载铜纳米团簇的单层结构具有高能量偏好。由于强烈的金属-氧化物相互作用,在 O 溢出后,单层团簇通过将电子转移到 Ce 4f 轨道而被高度氧化。进一步发现,水煤气变换反应在负载的铜单层上比在铜-二氧化铈外围更有利地发生,其中现场氧和相邻的氧化铜位点占据催化活性位点,协同促进水的生成。解离和羧基形成。目前的工作为强金属-载体相互作用及其在催化反应中的作用提供了机理见解,这可能为合理设计具有良好稳定性、分散性和催化活性的金属氧化物催化剂铺平道路。















































 京公网安备 11010802027423号
京公网安备 11010802027423号