当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition-Metal-Catalyzed Asymmetric Couplings of α-Aminoalkyl Fragments to Access Chiral Alkylamines
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-05-19 , DOI: 10.1021/acscatal.1c01545 Xiaomei Wu 1 , Jiangtao Ren 1 , Zhihui Shao 1 , Xiaodong Yang 1 , Deyun Qian 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-05-19 , DOI: 10.1021/acscatal.1c01545 Xiaomei Wu 1 , Jiangtao Ren 1 , Zhihui Shao 1 , Xiaodong Yang 1 , Deyun Qian 1
Affiliation
|
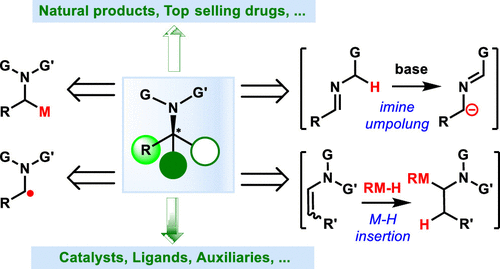
Enantiomerically pure alkylamines are pivotal structural elements in a broad range of pharmaceuticals, agrochemicals, natural products, and chemical building blocks. The development of useful methodologies for the preparation of these alkyamines is one of the central and long-standing challenges facing synthetic chemistry. In recent years, transition-metal-catalyzed enantioselective coupling reactions of α-aminoalkyl species have emerged as appealing C–C bond-forming approaches for the construction of chiral alkylamines. This useful technique is remarkable for its substantial advantages in functional group compatibility, substrate scope, molecular complexity, and synthetic practicality, which provides complementarity and orthogonality to traditional metal-catalyzed approaches to chiral amine synthesis. This Review underscores recent advances in transition-metal-catalyzed asymmetric alkylamine bond forming reactions organized by the type of key α-aminoalkyl intermediates, including (1) couplings of α-aminoalkyl-metal species, (2) couplings of α-aminoalkyl radicals, (3) couplings of imines with carbon electrophiles, and (4) couplings of enamines mediated by M–H species. In addition, the synthetic practicalities of such remarkable tools have also been highlighted by the total syntheses of selected natural and biologically interesting molecules and the stereoselective assembly of challenging target compounds.
中文翻译:

α-氨基烷基片段的过渡金属催化不对称偶联获得手性烷基胺
对映异构纯的烷基胺是广泛的药物、农用化学品、天然产品和化学构件中的关键结构元素。开发用于制备这些烷基胺的有用方法是合成化学面临的核心和长期挑战之一。近年来,过渡金属催化的α-氨基烷基物种的对映选择性偶联反应已成为构建手性烷基胺的有吸引力的C-C键形成方法。这种有用的技术因其在官能团兼容性、底物范围、分子复杂性和合成实用性方面的巨大优势而引人注目,它为传统的金属催化手性胺合成方法提供了互补性和正交性。本综述强调了过渡金属催化的不对称烷基胺键形成反应的最新进展,这些反应由关键的 α-氨基烷基中间体类型组织,包括 (1) α-氨基烷基-金属物种的偶联,(2) α-氨基烷基自由基的偶联, (3) 亚胺与碳亲电子试剂的偶联,以及 (4) 由 M-H 物种介导的烯胺偶联。此外,选定的天然和生物学上有趣的分子的全合成以及具有挑战性的目标化合物的立体选择性组装也突出了此类非凡工具的合成实用性。(3) 亚胺与碳亲电子试剂的偶联,以及 (4) 由 M-H 物种介导的烯胺偶联。此外,选定的天然和生物学上有趣的分子的全合成以及具有挑战性的目标化合物的立体选择性组装也突出了此类非凡工具的合成实用性。(3) 亚胺与碳亲电子试剂的偶联,以及 (4) 由 M-H 物种介导的烯胺偶联。此外,选定的天然和生物学上有趣的分子的全合成以及具有挑战性的目标化合物的立体选择性组装也突出了此类非凡工具的合成实用性。
更新日期:2021-06-04
中文翻译:

α-氨基烷基片段的过渡金属催化不对称偶联获得手性烷基胺
对映异构纯的烷基胺是广泛的药物、农用化学品、天然产品和化学构件中的关键结构元素。开发用于制备这些烷基胺的有用方法是合成化学面临的核心和长期挑战之一。近年来,过渡金属催化的α-氨基烷基物种的对映选择性偶联反应已成为构建手性烷基胺的有吸引力的C-C键形成方法。这种有用的技术因其在官能团兼容性、底物范围、分子复杂性和合成实用性方面的巨大优势而引人注目,它为传统的金属催化手性胺合成方法提供了互补性和正交性。本综述强调了过渡金属催化的不对称烷基胺键形成反应的最新进展,这些反应由关键的 α-氨基烷基中间体类型组织,包括 (1) α-氨基烷基-金属物种的偶联,(2) α-氨基烷基自由基的偶联, (3) 亚胺与碳亲电子试剂的偶联,以及 (4) 由 M-H 物种介导的烯胺偶联。此外,选定的天然和生物学上有趣的分子的全合成以及具有挑战性的目标化合物的立体选择性组装也突出了此类非凡工具的合成实用性。(3) 亚胺与碳亲电子试剂的偶联,以及 (4) 由 M-H 物种介导的烯胺偶联。此外,选定的天然和生物学上有趣的分子的全合成以及具有挑战性的目标化合物的立体选择性组装也突出了此类非凡工具的合成实用性。(3) 亚胺与碳亲电子试剂的偶联,以及 (4) 由 M-H 物种介导的烯胺偶联。此外,选定的天然和生物学上有趣的分子的全合成以及具有挑战性的目标化合物的立体选择性组装也突出了此类非凡工具的合成实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号