当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed Denitrogenative Diazole–Triazole Coupling toward Aza-Bridged Structures and Imidazole-Based Chelating Ligands
Organic Letters ( IF 4.9 ) Pub Date : 2021-05-17 , DOI: 10.1021/acs.orglett.1c01092 Julia O Strelnikova 1 , Alexander N Koronatov 1 , Nikolai V Rostovskii 1 , Alexander F Khlebnikov 1 , Olesya V Khoroshilova 1 , Mariya A Kryukova 1 , Mikhail S Novikov 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-05-17 , DOI: 10.1021/acs.orglett.1c01092 Julia O Strelnikova 1 , Alexander N Koronatov 1 , Nikolai V Rostovskii 1 , Alexander F Khlebnikov 1 , Olesya V Khoroshilova 1 , Mariya A Kryukova 1 , Mikhail S Novikov 1
Affiliation
|
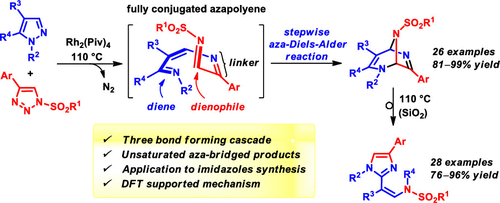
1,4,8-Triazaocta-1,3,5,7-tetraenes, generated in situ by Rh2(Piv)4-catalyzed denitrogenative coupling of pyrazoles with 1-sulfonyl-1,2,3-triazoles, smoothly form 2,6,8-triazabicyclo[3.2.1]octa-3,6-dienes via intramolecular aza-Diels–Alder cycloaddition. This domino reaction, combined with the subsequent thermal or acid-catalyzed rearrangement of the cycloadducts, provides direct and flexible access to N-sulfonylated (Z)-2-(2-aminovinyl)imidazoles.
中文翻译:

铑催化脱氮二唑-三唑偶联到氮杂桥结构和咪唑基螯合配体
1,4,8-Triazaocta-1,3,5,7-tetraenes,由 Rh 2 (Piv) 4催化的吡唑与 1-磺酰基-1,2,3-三唑的脱氮偶联原位生成,顺利形成 2 ,6,8-三氮杂双环[3.2.1]octa-3,6-二烯通过分子内氮杂-Diels-Alder环加成反应。这种多米诺反应与随后的环加合物的热或酸催化重排相结合,提供了直接和灵活地获得N-磺酰化 ( Z )-2-(2-氨基乙烯基)咪唑的途径。
更新日期:2021-06-04
中文翻译:

铑催化脱氮二唑-三唑偶联到氮杂桥结构和咪唑基螯合配体
1,4,8-Triazaocta-1,3,5,7-tetraenes,由 Rh 2 (Piv) 4催化的吡唑与 1-磺酰基-1,2,3-三唑的脱氮偶联原位生成,顺利形成 2 ,6,8-三氮杂双环[3.2.1]octa-3,6-二烯通过分子内氮杂-Diels-Alder环加成反应。这种多米诺反应与随后的环加合物的热或酸催化重排相结合,提供了直接和灵活地获得N-磺酰化 ( Z )-2-(2-氨基乙烯基)咪唑的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号