International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2021-05-15 , DOI: 10.1016/j.ijhydene.2021.04.080 Darshil Chodvadiya , Narayan N. Som , Prafulla K. Jha , Brahmananda Chakraborty
|
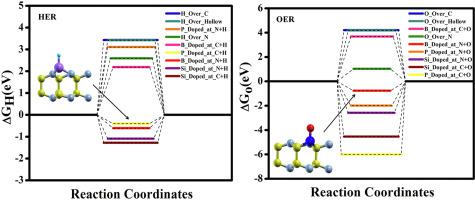
Employing the Density Functional Theory investigations, we have designed 2D α-CN with the dopants P, Si and B as catalyst for HER and OER activities. Doping of P and B over α-CN modifies its electronic properties and reduces band gap (3.78 eV) of α-CN to the required band gap for HER and OER activities. The modification of electronic properties is discussed by the analysis of partial density of states, Löwdin charge and charge density plot. To understand HER and OER activities better, we computed Gibbs free energy change after adsorption of H/O in various doped α-CN systems. We observe that the P doping at C site and B doping at N site of α-CN are best suited for HER and OER respectively. The HER (OER) activity increases by 88.33% (29.35%) for P doped at C site (B doped at N site) of α-CN in comparison to pristine α-CN.
中文翻译:

通过 B、Si 和 P 掺杂提高二维 α-CN 对析氢和析氧反应的催化活性
利用密度泛函理论研究,我们设计了 2D α-CN,掺杂剂 P、Si 和 B 作为 HER 和 OER 活性的催化剂。在 α-CN 上掺杂 P 和 B 会改变其电子特性,并将 α-CN 的带隙 (3.78 eV) 降低到 HER 和 OER 活性所需的带隙。通过分析部分态密度、Löwdin 电荷和电荷密度图来讨论电子特性的修改。为了更好地了解 HER 和 OER 活性,我们计算了在各种掺杂的 α-CN 体系中吸附 H/O 后的吉布斯自由能变化。我们观察到 α-CN 的 C 位的 P 掺杂和 N 位的 B 掺杂分别最适合 HER 和 OER。与原始 α-CN 相比,在 α-CN 的 C 位(在 N 位掺杂 B)的 P 掺杂的 HER(OER)活性增加了 88.33%(29.35%)。































 京公网安备 11010802027423号
京公网安备 11010802027423号