当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Biological Activity of Novel Pyrazol-5-yl-benzamide Derivatives as Potential Succinate Dehydrogenase Inhibitors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-05-14 , DOI: 10.1021/acs.jafc.0c08094 Wei Wang 1 , Jianhua Wang 1 , Furan Wu 1 , Huan Zhou 1 , Dan Xu 2, 3 , Gong Xu 1, 3
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-05-14 , DOI: 10.1021/acs.jafc.0c08094 Wei Wang 1 , Jianhua Wang 1 , Furan Wu 1 , Huan Zhou 1 , Dan Xu 2, 3 , Gong Xu 1, 3
Affiliation
|
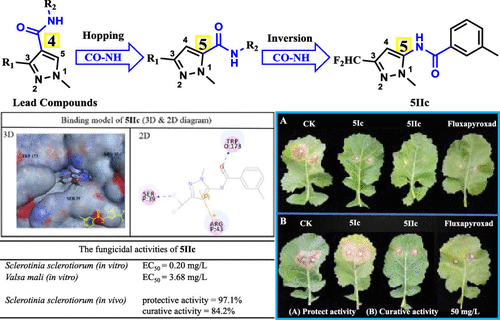
To promote the discovery and development of new fungicides, a series of novel pyrazol-5-yl-benzamide derivatives were designed, synthesized by hopping and inversion of amide groups of pyrazole-4-carboxamides, and evaluated for their antifungal activities. The bioassay data revealed that compound 5IIc exhibited an excellent in vitro activity against Sclerotinia sclerotiorum with an EC50 value of 0.20 mg/L, close to that of commercial fungicide Fluxapyroxad (EC50 = 0.12 mg/L) and Boscalid (EC50 = 0.11 mg/L). For Valsa mali, compound 5IIc (EC50 = 3.68 mg/L) showed a significantly higher activity than Fluxapyroxad (EC50 = 12.67 mg/L) and Boscalid (EC50 = 14.83 mg/L). In addition, in vivo experiments proved that compound 5IIc has an excellent protective fungicidal activity with an inhibitory rate of 97.1% against S. sclerotiorum at 50 mg/L, while the positive control Fluxapyroxad showed a 98.6% inhibitory effect. The molecular docking simulation revealed that compound 5IIc interact with TRP173, SER39, and ARG43 of succinate dehydrogenase (SDH) through a hydrogen bond and p−π interaction, which could explain the probable mechanism of the action between compound 5IIc and target protein. Also, the SDH enzymatic inhibition assay was carried out to further validate its mode of action. These results demonstrate that compound 5IIc could be a promising fungicide candidate and provide a valuable reference for further investigation.
中文翻译:

新型吡唑-5-基苯甲酰胺衍生物的合成及其生物活性作为潜在的琥珀酸脱氢酶抑制剂
为了促进新型杀菌剂的发现和开发,设计了一系列新型的吡唑-5-基-苯甲酰胺衍生物,通过对吡唑-4-羧酰胺的酰胺基进行跳变和转化合成,并对其抗真菌活性进行了评估。生物测定数据表明,化合物5IIc对菌核菌表现出优异的体外活性,EC 50值为0.20 mg / L,接近商业杀菌剂Fluxapyroxad(EC 50 = 0.12 mg / L)和Boscalid(EC 50 = 0.11)。毫克/升)。对于Valsa mali,化合物5IIc(EC 50= 3.68 mg / L)的活性明显高于Fluxapyroxad(EC 50 = 12.67 mg / L)和Boscalid(EC 50 = 14.83 mg / L)。另外,体内实验证明化合物5IIc具有优异的保护性杀真菌活性,在50mg / L下对核盘菌的抑制率为97.1%,而阳性对照Fluxapyroxad表现出98.6%的抑制作用。分子对接模拟显示化合物5IIc通过氢键和p-π相互作用与琥珀酸脱氢酶(SDH)的TRP173,SER39和ARG43相互作用,这可能解释了化合物之间作用的可能机理5IIc和靶蛋白。此外,进行了SDH酶抑制试验,以进一步验证其作用方式。这些结果表明,化合物5IIc可能是有希望的杀真菌剂候选物,并为进一步研究提供了有价值的参考。
更新日期:2021-05-26
中文翻译:

新型吡唑-5-基苯甲酰胺衍生物的合成及其生物活性作为潜在的琥珀酸脱氢酶抑制剂
为了促进新型杀菌剂的发现和开发,设计了一系列新型的吡唑-5-基-苯甲酰胺衍生物,通过对吡唑-4-羧酰胺的酰胺基进行跳变和转化合成,并对其抗真菌活性进行了评估。生物测定数据表明,化合物5IIc对菌核菌表现出优异的体外活性,EC 50值为0.20 mg / L,接近商业杀菌剂Fluxapyroxad(EC 50 = 0.12 mg / L)和Boscalid(EC 50 = 0.11)。毫克/升)。对于Valsa mali,化合物5IIc(EC 50= 3.68 mg / L)的活性明显高于Fluxapyroxad(EC 50 = 12.67 mg / L)和Boscalid(EC 50 = 14.83 mg / L)。另外,体内实验证明化合物5IIc具有优异的保护性杀真菌活性,在50mg / L下对核盘菌的抑制率为97.1%,而阳性对照Fluxapyroxad表现出98.6%的抑制作用。分子对接模拟显示化合物5IIc通过氢键和p-π相互作用与琥珀酸脱氢酶(SDH)的TRP173,SER39和ARG43相互作用,这可能解释了化合物之间作用的可能机理5IIc和靶蛋白。此外,进行了SDH酶抑制试验,以进一步验证其作用方式。这些结果表明,化合物5IIc可能是有希望的杀真菌剂候选物,并为进一步研究提供了有价值的参考。






























 京公网安备 11010802027423号
京公网安备 11010802027423号