当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Structure–Activity Relationships, and In Vivo Evaluation of Novel Tetrahydropyran-Based Thiodisaccharide Mimics as Galectin-3 Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-05-14 , DOI: 10.1021/acs.jmedchem.0c02001 Li Xu 1 , Richard A Hartz 1 , Brett R Beno 2 , Kaushik Ghosh 3 , Jinal K Shukla 3 , Amit Kumar 3 , Dipal Patel 4 , Narasimharaju Kalidindi 3 , Nadine Lemos 3 , Shashyendra Singh Gautam 3 , Anoop Kumar 3 , Bruce A Ellsworth 1 , Devang Shah 3 , Harinath Sale 3 , Dong Cheng 5 , Alicia Regueiro-Ren 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-05-14 , DOI: 10.1021/acs.jmedchem.0c02001 Li Xu 1 , Richard A Hartz 1 , Brett R Beno 2 , Kaushik Ghosh 3 , Jinal K Shukla 3 , Amit Kumar 3 , Dipal Patel 4 , Narasimharaju Kalidindi 3 , Nadine Lemos 3 , Shashyendra Singh Gautam 3 , Anoop Kumar 3 , Bruce A Ellsworth 1 , Devang Shah 3 , Harinath Sale 3 , Dong Cheng 5 , Alicia Regueiro-Ren 1
Affiliation
|
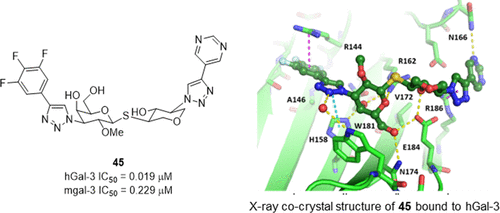
Galectin-3 is a member of a family of β-galactoside-binding proteins. A substantial body of literature reports that galectin-3 plays important roles in cancer, inflammation, and fibrosis. Small-molecule galectin-3 inhibitors, which are generally lactose or galactose-based derivatives, have the potential to be valuable disease-modifying agents. In our efforts to identify novel galectin-3 disaccharide mimics to improve drug-like properties, we found that one of the monosaccharide subunits can be replaced with a suitably functionalized tetrahydropyran ring. Optimization of the structure–activity relationships around the tetrahydropyran-based scaffold led to the discovery of potent galectin-3 inhibitors. Compounds 36, 40, and 45 were selected for further in vivo evaluation. The synthesis, structure–activity relationships, and in vivo evaluation of novel tetrahydropyran-based galectin-3 inhibitors are described.
中文翻译:

合成,结构-活性之间的关系,和体内的新型基于四氢吡喃的巯基糖模拟物作为Galectin-3抑制剂的体内评价。
Galectin-3是β-半乳糖苷结合蛋白家族的成员。大量文献报道,galectin-3在癌症,炎症和纤维化中起重要作用。小分子半乳糖凝集素3抑制剂通常是乳糖或半乳糖基衍生物,具有成为有价值的疾病缓解剂的潜力。在我们努力确定新型半乳糖凝集素3二糖模拟物以改善药物样特性的过程中,我们发现单糖亚基之一可以被适当官能化的四氢吡喃环取代。优化基于四氢吡喃的支架周围的结构-活性关系导致了有效的galectin-3抑制剂的发现。化合物36,40,和45选择用于进一步的体内评估。描述了新型的基于四氢吡喃的galectin-3抑制剂的合成,结构-活性关系以及体内评估。
更新日期:2021-05-27
中文翻译:

合成,结构-活性之间的关系,和体内的新型基于四氢吡喃的巯基糖模拟物作为Galectin-3抑制剂的体内评价。
Galectin-3是β-半乳糖苷结合蛋白家族的成员。大量文献报道,galectin-3在癌症,炎症和纤维化中起重要作用。小分子半乳糖凝集素3抑制剂通常是乳糖或半乳糖基衍生物,具有成为有价值的疾病缓解剂的潜力。在我们努力确定新型半乳糖凝集素3二糖模拟物以改善药物样特性的过程中,我们发现单糖亚基之一可以被适当官能化的四氢吡喃环取代。优化基于四氢吡喃的支架周围的结构-活性关系导致了有效的galectin-3抑制剂的发现。化合物36,40,和45选择用于进一步的体内评估。描述了新型的基于四氢吡喃的galectin-3抑制剂的合成,结构-活性关系以及体内评估。































 京公网安备 11010802027423号
京公网安备 11010802027423号