Chem ( IF 19.1 ) Pub Date : 2021-05-13 , DOI: 10.1016/j.chempr.2021.04.005 Ze-Shui Liu , Pei-Pei Xie , Yu Hua , Chenggui Wu , Yuanyuan Ma , Jiangwei Chen , Hong-Gang Cheng , Xin Hong , Qianghui Zhou
|
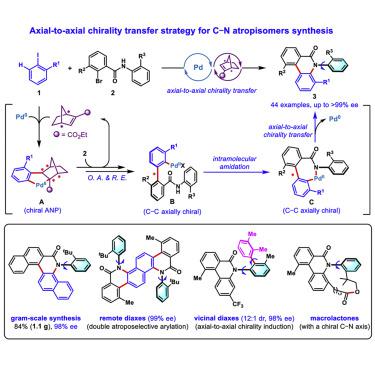
C–N axially chiral skeletons are ubiquitous in bioactive natural products, pharmaceuticals, and chiral ligands. However, their atroposelective synthesis remains a formidable challenge because of their innate low configurational stability compared with that of well-developed C–C atropisomers. Herein, we report a general and efficient method for accessing C–N atropisomers through an axial-to-axial chirality transfer strategy based on palladium/chiral norbornene cooperative catalysis. The obtained C–N axial chirality originates from the preformed transient C–C axial chirality with high fidelity. A variety of C–N axially chiral phenanthridinones are obtained in excellent enantioselectivities (44 examples, up to >99% ee). This method can be applied for the construction of two stereogenic axes via double atroposelective C–H arylation or further transformation of the products via axial-to-axial diastereoinduction. Additionally, the reaction mechanism and the chirality transfer process are elucidated by density functional theory calculations.
中文翻译:

C-N轴手性的atroposelective构建的轴到轴手性转移策略
C-N 轴向手性骨架在生物活性天然产物、药物和手性配体中无处不在。然而,它们的阻转选择性合成仍然是一个艰巨的挑战,因为与成熟的 C-C 阻转异构体相比,它们的先天低构型稳定性。在此,我们报告了一种通过基于钯/手性降冰片烯协同催化的轴-轴手性转移策略获取 C-N 阻转异构体的通用且有效的方法。获得的 C-N 轴向手性源于具有高保真度的预先形成的瞬态 C-C 轴向手性。以优异的对映选择性获得了多种 C-N 轴向手性菲啶酮(44 个实例,高达 >99% ee)。该方法可用于通过双 atroposelective C-H 芳基化或通过轴对轴非对映诱导进一步转化产物来构建两个立体轴。此外,通过密度泛函理论计算阐明了反应机理和手性转移过程。































 京公网安备 11010802027423号
京公网安备 11010802027423号