当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Enzymatic Analysis of Tumor-Targeted Antifolates That Inhibit Glycinamide Ribonucleotide Formyltransferase
Biochemistry ( IF 2.9 ) Pub Date : 2016-08-03 00:00:00 , DOI: 10.1021/acs.biochem.6b00412
Siobhan M Deis , Arpit Doshi 1 , Zhanjun Hou 2, 3 , Larry H Matherly 2, 3, 4 , Aleem Gangjee 1 , Charles E Dann
Biochemistry ( IF 2.9 ) Pub Date : 2016-08-03 00:00:00 , DOI: 10.1021/acs.biochem.6b00412
Siobhan M Deis , Arpit Doshi 1 , Zhanjun Hou 2, 3 , Larry H Matherly 2, 3, 4 , Aleem Gangjee 1 , Charles E Dann
Affiliation
|
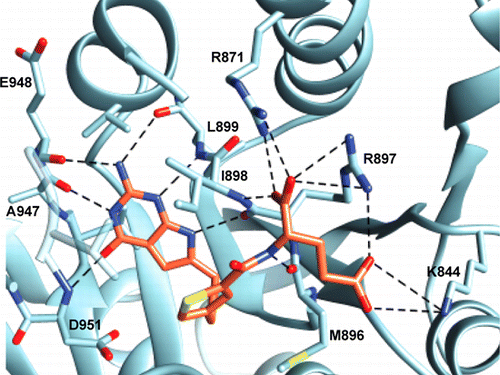
Pemetrexed and methotrexate are antifolates used for cancer chemotherapy and inflammatory diseases. These agents have toxic side effects resulting, in part, from nonspecific cellular transport by the reduced folate carrier (RFC), a ubiquitously expressed facilitative transporter. We previously described 2-amino-4-oxo-6-substituted pyrrolo[2,3-d]pyrimidine antifolates with modifications of the side chain linker and aromatic ring that are poor substrates for RFC but are efficiently transported via folate receptors (FRs) and the proton-coupled folate transporter (PCFT). These targeted antifolates are cytotoxic in vitro toward FR- and PCFT-expressing tumor cells and in vivo with human tumor xenografts in immune-compromised mice, reflecting selective cellular uptake. Antitumor efficacy is due to inhibition of glycinamide ribonucleotide (GAR) formyltransferase (GARFTase) activity in de novo synthesis of purine nucleotides. This study used purified human GARFTase (formyltransferase domain) to assess in vitro inhibition by eight novel thieno- and pyrrolo[2,3-d]pyrimidine antifolates. Seven analogues (AGF23, AGF71, AGF94, AGF117, AGF118, AGF145, and AGF147) inhibited GARFTase with Ki values in the low- to mid-nanomolar concentration range, whereas AGF50 inhibited GARFTase with micromolar potency similar to that of PMX. On the basis of crystal structures of ternary complexes with GARFTase, β-GAR, and the monoglutamyl antifolates, differences in inhibitory potencies correlated well with antifolate binding and the positions of the terminal carboxylates. Our data provide a mechanistic basis for differences in inhibitory potencies between these novel antifolates and a framework for future structure-based drug design. These analogues could be more efficacious than clinically used antifolates, reflecting their selective cellular uptake by FRs and PCFT and potent GARFTase inhibition.
中文翻译:

抑制甘氨酰胺核糖核苷酸甲酰基转移酶的肿瘤靶向抗叶酸药物的结构和酶学分析
培美曲塞和甲氨蝶呤是用于癌症化疗和炎症性疾病的抗叶酸剂。这些药物具有毒副作用,部分原因是还原叶酸载体(RFC)(一种普遍表达的易化转运蛋白)的非特异性细胞转运所致。我们之前描述了 2-氨基-4-氧代-6-取代的吡咯并[2,3- d ]嘧啶抗叶酸剂,其侧链接头和芳香环经过修饰,是 RFC 的不良底物,但可通过叶酸受体 (FR) 有效转运和质子耦合叶酸转运蛋白(PCFT)。这些靶向抗叶酸剂在体外对表达 FR 和 PCFT 的肿瘤细胞具有细胞毒性,在体内对免疫受损小鼠的人类肿瘤异种移植物具有细胞毒性,反映了选择性细胞摄取。抗肿瘤功效是由于抑制了嘌呤核苷酸从头合成中的甘氨酰胺核糖核苷酸 (GAR) 甲酰基转移酶 (GARFTase) 活性。本研究使用纯化的人 GARFTase(甲酰基转移酶结构域)来评估八种新型噻吩并和吡咯并[2,3- d ]嘧啶抗叶酸剂的体外抑制作用。七种类似物(AGF23、AGF71、AGF94、AGF117、AGF118、AGF145 和 AGF147)以低至中纳摩尔浓度范围内的K值抑制 GARFTase,而 AGF50 以与 PMX 相似的微摩尔效力抑制 GARFTase。根据 GARFTase、β-GAR 和单谷氨酰抗叶酸剂三元复合物的晶体结构,抑制效力的差异与抗叶酸剂结合和末端羧酸盐的位置密切相关。 我们的数据为这些新型抗叶酸剂之间的抑制效力差异提供了机制基础,并为未来基于结构的药物设计提供了框架。这些类似物可能比临床使用的抗叶酸剂更有效,这反映了它们通过 FR 和 PCFT 的选择性细胞摄取以及有效的 GARFTase 抑制。
更新日期:2016-08-03
中文翻译:

抑制甘氨酰胺核糖核苷酸甲酰基转移酶的肿瘤靶向抗叶酸药物的结构和酶学分析
培美曲塞和甲氨蝶呤是用于癌症化疗和炎症性疾病的抗叶酸剂。这些药物具有毒副作用,部分原因是还原叶酸载体(RFC)(一种普遍表达的易化转运蛋白)的非特异性细胞转运所致。我们之前描述了 2-氨基-4-氧代-6-取代的吡咯并[2,3- d ]嘧啶抗叶酸剂,其侧链接头和芳香环经过修饰,是 RFC 的不良底物,但可通过叶酸受体 (FR) 有效转运和质子耦合叶酸转运蛋白(PCFT)。这些靶向抗叶酸剂在体外对表达 FR 和 PCFT 的肿瘤细胞具有细胞毒性,在体内对免疫受损小鼠的人类肿瘤异种移植物具有细胞毒性,反映了选择性细胞摄取。抗肿瘤功效是由于抑制了嘌呤核苷酸从头合成中的甘氨酰胺核糖核苷酸 (GAR) 甲酰基转移酶 (GARFTase) 活性。本研究使用纯化的人 GARFTase(甲酰基转移酶结构域)来评估八种新型噻吩并和吡咯并[2,3- d ]嘧啶抗叶酸剂的体外抑制作用。七种类似物(AGF23、AGF71、AGF94、AGF117、AGF118、AGF145 和 AGF147)以低至中纳摩尔浓度范围内的K值抑制 GARFTase,而 AGF50 以与 PMX 相似的微摩尔效力抑制 GARFTase。根据 GARFTase、β-GAR 和单谷氨酰抗叶酸剂三元复合物的晶体结构,抑制效力的差异与抗叶酸剂结合和末端羧酸盐的位置密切相关。 我们的数据为这些新型抗叶酸剂之间的抑制效力差异提供了机制基础,并为未来基于结构的药物设计提供了框架。这些类似物可能比临床使用的抗叶酸剂更有效,这反映了它们通过 FR 和 PCFT 的选择性细胞摄取以及有效的 GARFTase 抑制。































 京公网安备 11010802027423号
京公网安备 11010802027423号