当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Au(I) Catalyzed Synthesis of Densely Substituted Pyrazolines and Dihydropyridines via Sequential Aza-Enyne Metathesis/6π-Electrocyclization
Organic Letters ( IF 4.9 ) Pub Date : 2021-05-12 , DOI: 10.1021/acs.orglett.1c01171 Kenji Sugimoto 1 , Shuto Kosuge 1 , Takae Sugita 1 , Yuka Miura 1 , Kiyoshi Tsuge 2 , Yuji Matsuya 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-05-12 , DOI: 10.1021/acs.orglett.1c01171 Kenji Sugimoto 1 , Shuto Kosuge 1 , Takae Sugita 1 , Yuka Miura 1 , Kiyoshi Tsuge 2 , Yuji Matsuya 1
Affiliation
|
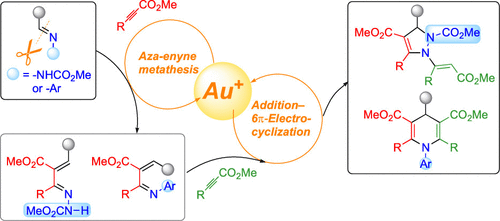
A gold(I) autotandem catalysis protocol is reported for the de novo synthesis of densely substituted pyrazolines and dihydropyridines from the corresponding imine derivatives in a highly regioselective fashion via a one-pot aza-enyne metathesis/6π-electrocyclization sequence. The substituents on the nitrogen atom of the imine perfectly control the reaction pathways from the pivotal 1-azabutadiene intermediate; thus, carbazates were converted into pyrazolines via 6π-electrocyclization of α,β-unsaturated hydrazones, while aryl imines provided dihydropyridines via 6π-electrocyclization of 3-azahexatrienes.
中文翻译:

Au(I)通过顺序氮杂-Enyne复分解/6π-电环化催化合成重取代的吡唑啉和二氢吡啶
报道了一种金(I)自动串联催化方案,用于通过一锅氮杂-烯炔复分解/6π-电环化序列,以高度区域选择性的方式从相应的亚胺衍生物从头合成密集取代的吡唑啉和二氢吡啶。亚胺氮原子上的取代基可以完美地控制来自关键的1-氮杂丁二烯中间体的反应路径;因此,氨基甲酸酯通过α,β-不饱和6的6π-电环化转化为吡唑啉,而芳基亚胺通过3-氮杂己三烯的6π-电环化提供二氢吡啶。
更新日期:2021-05-22
中文翻译:

Au(I)通过顺序氮杂-Enyne复分解/6π-电环化催化合成重取代的吡唑啉和二氢吡啶
报道了一种金(I)自动串联催化方案,用于通过一锅氮杂-烯炔复分解/6π-电环化序列,以高度区域选择性的方式从相应的亚胺衍生物从头合成密集取代的吡唑啉和二氢吡啶。亚胺氮原子上的取代基可以完美地控制来自关键的1-氮杂丁二烯中间体的反应路径;因此,氨基甲酸酯通过α,β-不饱和6的6π-电环化转化为吡唑啉,而芳基亚胺通过3-氮杂己三烯的6π-电环化提供二氢吡啶。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号