当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Varkud Satellite Ribozyme: A Thirty-Year Journey through Biochemistry, Crystallography, and Computation
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-05-11 , DOI: 10.1021/acs.accounts.1c00052 Saurja DasGupta 1, 2, 3, 4 , Joseph A Piccirilli
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-05-11 , DOI: 10.1021/acs.accounts.1c00052 Saurja DasGupta 1, 2, 3, 4 , Joseph A Piccirilli
Affiliation
|
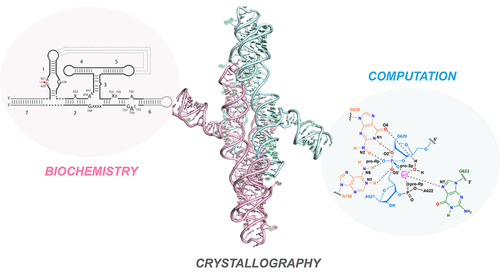
The discovery of catalytic RNAs or ribozymes introduced a new class of enzymes to biology. In addition to their increasingly important roles in modern life, ribozymes are key players in the RNA World hypothesis, which posits that life started or flourished with RNA supporting both genetic and enzymatic functions. Therefore, investigations into the mechanisms of ribozyme function provide an exciting opportunity to examine the foundational principles of biological catalysis. Ribozymes are also attractive model systems to investigate the relationship between structure and function in RNA. Endonucleolytic ribozymes represent the largest class of catalytic RNA, of which the Varkud satellite (VS) ribozyme is structurally the most complex. The last ribozyme to be discovered by accident, the VS ribozyme had eluded structural determination for over two decades. When we solved the first crystal structures of the VS ribozyme, an extensive body of biochemical and biophysical data had accumulated over the years with which we could evaluate the functional relevance of the structure. Conversely, the structures provided a new perspective from which to reexamine the functional data and test new hypotheses. The VS ribozyme is organized in a modular fashion where independently folding domains assemble into the active conformation of the ribozyme via three-way junctions. Structures of the VS ribozyme in complex with its substrate at different stages of activation enabled us to map the structural reorganization of the substrate that must precede catalysis. In addition to defining the global architecture of the RNA, the essential interactions between the substrate and catalytic domains, and the rearrangements in the substrate prior to catalysis, these structures provided detailed snapshots of the ribozyme active site, revealing potential catalytic interactions. High resolution structures of the active site bolstered the view that the catalytic mechanism involved nucleobase-mediated general acid–base catalysis and uncovered additional catalytic interactions between the cleavage site and catalytic residues. Informed by the crystal structures of the VS ribozyme, an integrated experimental and computational approach identified the key players and essential interactions that define the active site of the ribozyme. This confluence of biochemical, structural, and computational studies revealed the catalytic mechanism of the ribozyme at unprecedented detail. Additionally, comparative analyses of the active site structures of the VS ribozyme and other nucleic acid-based endoribonucleases revealed common architectural motifs and strikingly similar catalytic strategies. In this Account, we document the progress of VS ribozyme research starting from its discovery and extending to the elucidation of its detailed catalytic mechanism 30 years later.
中文翻译:

Varkud 卫星核酶:生物化学、晶体学和计算的三十年之旅
催化 RNA 或核酶的发现为生物学引入了一类新的酶。核酶除了在现代生活中发挥着日益重要的作用外,还是RNA世界假说的关键参与者,该假说认为生命的开始或繁荣是由支持遗传和酶功能的RNA开始的。因此,对核酶功能机制的研究为研究生物催化的基本原理提供了令人兴奋的机会。核酶也是研究 RNA 结构和功能之间关系的有吸引力的模型系统。核酸内切核酶代表最大类别的催化 RNA,其中 Varkud 卫星 (VS) 核酶结构最复杂。 VS 核酶是最后一个偶然发现的核酶,二十多年来一直未能确定其结构。当我们解析出 VS 核酶的第一个晶体结构时,多年来积累了大量的生化和生物物理数据,我们可以利用这些数据来评估该结构的功能相关性。相反,这些结构提供了一个新的视角来重新检查功能数据并测试新的假设。 VS 核酶以模块化方式组织,其中独立折叠结构域通过三路连接组装成核酶的活性构象。 VS 核酶与其底物在不同激活阶段复合的结构使我们能够绘制催化之前必须进行的底物的结构重组。 除了定义 RNA 的整体结构、底物和催化结构域之间的基本相互作用以及催化前底物的重排之外,这些结构还提供了核酶活性位点的详细快照,揭示了潜在的催化相互作用。活性位点的高分辨率结构支持了催化机制涉及核碱基介导的一般酸碱催化的观点,并揭示了裂解位点和催化残基之间的额外催化相互作用。根据 VS 核酶的晶体结构,综合实验和计算方法确定了定义核酶活性位点的关键参与者和基本相互作用。生物化学、结构和计算研究的融合以前所未有的细节揭示了核酶的催化机制。此外,对 VS 核酶和其他基于核酸的核糖核酸内切酶活性位点结构的比较分析揭示了共同的结构基序和惊人相似的催化策略。在这篇文章中,我们记录了VS核酶的研究进展,从它的发现开始,一直延伸到30年后对其详细催化机制的阐明。
更新日期:2021-06-01
中文翻译:

Varkud 卫星核酶:生物化学、晶体学和计算的三十年之旅
催化 RNA 或核酶的发现为生物学引入了一类新的酶。核酶除了在现代生活中发挥着日益重要的作用外,还是RNA世界假说的关键参与者,该假说认为生命的开始或繁荣是由支持遗传和酶功能的RNA开始的。因此,对核酶功能机制的研究为研究生物催化的基本原理提供了令人兴奋的机会。核酶也是研究 RNA 结构和功能之间关系的有吸引力的模型系统。核酸内切核酶代表最大类别的催化 RNA,其中 Varkud 卫星 (VS) 核酶结构最复杂。 VS 核酶是最后一个偶然发现的核酶,二十多年来一直未能确定其结构。当我们解析出 VS 核酶的第一个晶体结构时,多年来积累了大量的生化和生物物理数据,我们可以利用这些数据来评估该结构的功能相关性。相反,这些结构提供了一个新的视角来重新检查功能数据并测试新的假设。 VS 核酶以模块化方式组织,其中独立折叠结构域通过三路连接组装成核酶的活性构象。 VS 核酶与其底物在不同激活阶段复合的结构使我们能够绘制催化之前必须进行的底物的结构重组。 除了定义 RNA 的整体结构、底物和催化结构域之间的基本相互作用以及催化前底物的重排之外,这些结构还提供了核酶活性位点的详细快照,揭示了潜在的催化相互作用。活性位点的高分辨率结构支持了催化机制涉及核碱基介导的一般酸碱催化的观点,并揭示了裂解位点和催化残基之间的额外催化相互作用。根据 VS 核酶的晶体结构,综合实验和计算方法确定了定义核酶活性位点的关键参与者和基本相互作用。生物化学、结构和计算研究的融合以前所未有的细节揭示了核酶的催化机制。此外,对 VS 核酶和其他基于核酸的核糖核酸内切酶活性位点结构的比较分析揭示了共同的结构基序和惊人相似的催化策略。在这篇文章中,我们记录了VS核酶的研究进展,从它的发现开始,一直延伸到30年后对其详细催化机制的阐明。













































 京公网安备 11010802027423号
京公网安备 11010802027423号