当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Selective Chemoenzymatic Modification on the Core Fucose of an Antibody Enhances Its Fcγ Receptor Affinity and ADCC Activity
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-05-12 , DOI: 10.1021/jacs.1c03174 Chao Li 1 , Gene Chong 2 , Guanghui Zong 1 , David A Knorr 3 , Stylianos Bournazos 3 , Asaminew Haile Aytenfisu 2 , Grace K Henry 1 , Jeffrey V Ravetch 3 , Alexander D MacKerell 2 , Lai-Xi Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-05-12 , DOI: 10.1021/jacs.1c03174 Chao Li 1 , Gene Chong 2 , Guanghui Zong 1 , David A Knorr 3 , Stylianos Bournazos 3 , Asaminew Haile Aytenfisu 2 , Grace K Henry 1 , Jeffrey V Ravetch 3 , Alexander D MacKerell 2 , Lai-Xi Wang 1
Affiliation
|
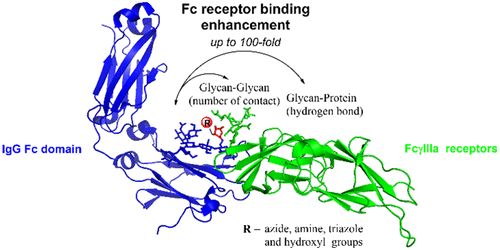
Fc glycosylation profoundly impacts the effector functions of antibodies and often dictates an antibody’s pro- or anti-inflammatory activities. It is well established that core fucosylation of the Fc domain N-glycans of an antibody significantly reduces its affinity for FcγRIIIa receptors and antibody-dependent cellular cytotoxicity (ADCC). Previous structural studies have suggested that the presence of a core fucose remarkably decreases the unique and favorable carbohydrate–carbohydrate interactions between the Fc and the receptor N-glycans, leading to reduced affinity. We report here that in contrast to natural core fucose, special site-specific modification on the core fucose could dramatically enhance the affinity of an antibody for FcγRIIIa. The site-selective modification was achieved through an enzymatic transfucosylation with a novel fucosidase mutant, which was shown to be able to use modified α-fucosyl fluoride as the donor substrate. We found that replacement of the core l-fucose with 6-azide- or 6-hydroxy-l-fucose (l-galactose) significantly enhanced the antibody’s affinity for FcγRIIIa receptors and substantially increased the ADCC activity. To understand the mechanism of the modified fucose-mediated affinity enhancement, we performed molecular dynamics simulations. Our data revealed that the number of glycan contacts between the Fc and the Fc receptor was increased by the selective core-fucose modifications, showing the importance of unique carbohydrate–carbohydrate interactions in achieving high FcγRIIIa affinity and ADCC activity of antibodies. Thus, the direct site-selective modification turns the adverse effect of the core fucose into a favorable force to promote the carbohydrate–carbohydrate interactions.
中文翻译:

抗体核心岩藻糖的位点选择性化学酶修饰增强其 Fcγ 受体亲和力和 ADCC 活性
Fc 糖基化深刻影响抗体的效应器功能,并经常决定抗体的促炎或抗炎活性。众所周知,抗体的 Fc 结构域N-聚糖的核心岩藻糖基化显着降低了其对 FcγRIIIa 受体的亲和力和抗体依赖性细胞毒性 (ADCC)。先前的结构研究表明,核心岩藻糖的存在显着降低了 Fc 和受体N之间独特且有利的碳水化合物-碳水化合物相互作用。-聚糖,导致亲和力降低。我们在此报告,与天然核心岩藻糖相比,对核心岩藻糖的特殊位点特异性修饰可以显着增强抗体对 FcγRIIIa 的亲和力。位点选择性修饰是通过用新型岩藻糖苷酶突变体进行酶促转岩藻糖基化来实现的,该突变体被证明能够使用修饰的 α-岩藻糖基氟化物作为供体底物。我们发现用 6-叠氮化物或 6-羟基-l-岩藻糖(l-岩藻糖)替代核心l-岩藻糖-galactose) 显着增强了抗体对 FcγRIIIa 受体的亲和力并显着增加了 ADCC 活性。为了了解修饰的岩藻糖介导的亲和力增强的机制,我们进行了分子动力学模拟。我们的数据显示,选择性核心-岩藻糖修饰增加了 Fc 和 Fc 受体之间的聚糖接触数量,表明独特的碳水化合物-碳水化合物相互作用在实现抗体的高 FcγRIIIa 亲和力和 ADCC 活性方面的重要性。因此,直接的位点选择性修饰将核心岩藻糖的不利影响转化为促进碳水化合物-碳水化合物相互作用的有利力量。
更新日期:2021-05-26
中文翻译:

抗体核心岩藻糖的位点选择性化学酶修饰增强其 Fcγ 受体亲和力和 ADCC 活性
Fc 糖基化深刻影响抗体的效应器功能,并经常决定抗体的促炎或抗炎活性。众所周知,抗体的 Fc 结构域N-聚糖的核心岩藻糖基化显着降低了其对 FcγRIIIa 受体的亲和力和抗体依赖性细胞毒性 (ADCC)。先前的结构研究表明,核心岩藻糖的存在显着降低了 Fc 和受体N之间独特且有利的碳水化合物-碳水化合物相互作用。-聚糖,导致亲和力降低。我们在此报告,与天然核心岩藻糖相比,对核心岩藻糖的特殊位点特异性修饰可以显着增强抗体对 FcγRIIIa 的亲和力。位点选择性修饰是通过用新型岩藻糖苷酶突变体进行酶促转岩藻糖基化来实现的,该突变体被证明能够使用修饰的 α-岩藻糖基氟化物作为供体底物。我们发现用 6-叠氮化物或 6-羟基-l-岩藻糖(l-岩藻糖)替代核心l-岩藻糖-galactose) 显着增强了抗体对 FcγRIIIa 受体的亲和力并显着增加了 ADCC 活性。为了了解修饰的岩藻糖介导的亲和力增强的机制,我们进行了分子动力学模拟。我们的数据显示,选择性核心-岩藻糖修饰增加了 Fc 和 Fc 受体之间的聚糖接触数量,表明独特的碳水化合物-碳水化合物相互作用在实现抗体的高 FcγRIIIa 亲和力和 ADCC 活性方面的重要性。因此,直接的位点选择性修饰将核心岩藻糖的不利影响转化为促进碳水化合物-碳水化合物相互作用的有利力量。

































 京公网安备 11010802027423号
京公网安备 11010802027423号