Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-05-09 , DOI: 10.1016/j.bmc.2021.116208 Osamu Kubo 1 , Kazuaki Takami 1 , Masahiro Kamaura 1 , Koji Watanabe 1 , Hirohisa Miyashita 1 , Shinichi Abe 1 , Kae Matsuda 1 , Yoshiyuki Tsujihata 1 , Tomoyuki Odani 2 , Shinji Iwasaki 3 , Tomoyuki Kitazaki 1 , Toshiki Murata 1 , Kenjiro Sato 1
|
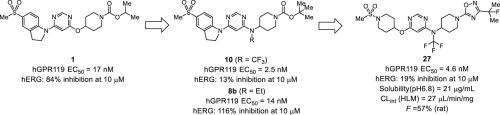
We undertook an optimization effort involving propan-2-yl 4-({6-[5-(methanesulfonyl)-2,3-dihydro-1H-indol-1-yl]pyrimidin-4-yl}oxy)piperidine-1-carboxylate 1, which we had previously discovered as a novel G protein-coupled receptor 119 (GPR119) agonist. To occupy a presumed hydrophobic space between the pyrimidine and piperidine rings in interaction with GPR119, we replaced the linker oxygen with nitrogen. Subsequently, the introduction of a substituent at the bridging nitrogen atom was explored. We found that the installation of N-trifluoromethyl group 10 not only enhanced GPR119 agonist activity but also considerably improved the human ether-à-go-go-related gene (hERG) inhibition profile. These improvements were not observed for non-fluorinated substituents, such as ethyl analog 8b. The next optimization effort focused on the exploration of a new surrogate structure for the indoline ring and the isosteric replacements of the piperidine N-Boc group to improve solubility, metabolic stability, and oral bioavailability. As a result, N-{1-[3-(2-fluoropropan-2-yl)-1,2,4-oxadiazol-5-yl]piperidin-4-yl}-6-{[1-(methanesulfonyl)piperidin-4-yl]oxy}-N-(trifluoromethyl)pyrimidin-4-amine (27) was identified as a potent and orally bioavailable GPR119 agonist. This compound augmented insulin secretion and effectively lowered plasma glucose excursion in a diabetic animal model after oral administration. In this study, we discuss the designs, syntheses, and biological activities of a novel series of N-(piperidin-4-yl)-N-(trifluoromethyl)pyrimidin-4-amine derivatives as GPR119 agonists, and to determine the distinctive effect of the N-trifluoromethyl group on hERG inhibition, we also discuss the conformational preference of representative compounds.
中文翻译:

新系列 GPR119 激动剂的发现:N-(Piperidin-4-yl)-N-(trifluoromethyl)pyrimidin-4-amine 衍生物的设计、合成和生物学评价
我们进行了涉及 propan-2-yl 4-({6-[5-(methanesulfonyl)-2,3-dihydro-1 H -indol-1-yl]pyrimidin-4-yl}oxy)piperidine-1 的优化工作-羧酸盐1,我们之前发现它是一种新型 G 蛋白偶联受体 119 (GPR119) 激动剂。为了在与 GPR119 相互作用的嘧啶和哌啶环之间占据一个假定的疏水空间,我们用氮替换了接头氧。随后,探索了在桥连氮原子上引入取代基。我们发现安装了N-三氟甲基10不仅增强了 GPR119 激动剂的活性,而且显着改善了人类 ether-à-go-go 相关基因 (hERG) 的抑制特性。对于非氟化取代基,例如乙基类似物8b,未观察到这些改进。下一个优化工作的重点是探索二氢吲哚环的新替代结构和哌啶N- Boc 基团的等排置换,以提高溶解度、代谢稳定性和口服生物利用度。结果,N -{1-[3-(2-fluoropropan-2-yl)-1,2,4-oxadiazol-5-yl]piperidin-4-yl}-6-{[1-(methanesulfonyl)哌啶-4-基]氧基} -N- (三氟甲基)嘧啶-4-胺( 27) 被鉴定为一种有效的口服生物可利用的 GPR119 激动剂。在口服给药后,该化合物在糖尿病动物模型中增加胰岛素分泌并有效降低血浆葡萄糖偏移。在本研究中,我们讨论了作为 GPR119 激动剂的一系列新型N -(piperidin-4-yl)- N -(trifluoromethyl)pyrimidin-4-amine 衍生物的设计、合成和生物活性,并确定其独特的作用所述的ñ上hERG抑制三氟甲基组,我们还讨论代表性的化合物的构象偏好。






























 京公网安备 11010802027423号
京公网安备 11010802027423号