当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Detection of Water Molecules on the Radical Transfer Pathway of Ribonucleotide Reductase by 17O Electron–Nuclear Double Resonance Spectroscopy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-05-06 , DOI: 10.1021/jacs.1c01359 Fabian Hecker 1 , JoAnne Stubbe 2 , Marina Bennati 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-05-06 , DOI: 10.1021/jacs.1c01359 Fabian Hecker 1 , JoAnne Stubbe 2 , Marina Bennati 1, 3
Affiliation
|
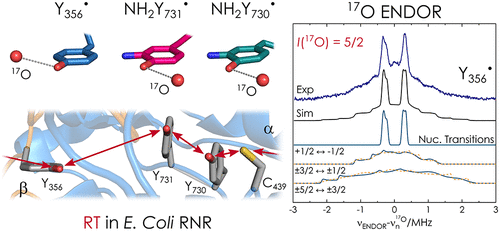
The role of water in biological proton-coupled electron transfer (PCET) is emerging as a key for understanding mechanistic details at atomic resolution. Here we demonstrate 17O high-frequency electron–nuclear double resonance (ENDOR) in conjunction with H217O-labeled protein buffer to establish the presence of ordered water molecules at three radical intermediates in an active enzyme complex, the α2β2E. coli ribonucleotide reductase. Our data give unambiguous evidence that all three, individually trapped, intermediates are hyperfine coupled to one water molecule with Tyr-O···17O distances in the range 2.8–3.1 Å. The availability of this structural information will allow for quantitative models of PCET in this prototype enzyme. The results also provide a spectroscopic signature for water H-bonded to a tyrosyl radical.
中文翻译:

17O电子核双共振谱检测核糖核苷酸还原酶自由基转移途径中的水分子
水在生物质子耦合电子转移(PCET)中的作用正在成为理解原子分辨率机制细节的关键。在这里,我们展示了17 O 高频电子核双共振 (ENDOR) 与 H 2 17 O 标记的蛋白质缓冲液相结合,以确定活性酶复合物 α 2 β 2中三个自由基中间体处有序水分子的存在大肠杆菌核糖核苷酸还原酶。我们的数据提供了明确的证据,表明所有三种单独捕获的中间体都与一个水分子超精细耦合,Tyr-O… 17 O 距离在 2.8–3.1 Å 范围内。该结构信息的可用性将允许在该原型酶中建立 PCET 的定量模型。结果还提供了与酪氨酰自由基键合的水的光谱特征。
更新日期:2021-05-19
中文翻译:

17O电子核双共振谱检测核糖核苷酸还原酶自由基转移途径中的水分子
水在生物质子耦合电子转移(PCET)中的作用正在成为理解原子分辨率机制细节的关键。在这里,我们展示了17 O 高频电子核双共振 (ENDOR) 与 H 2 17 O 标记的蛋白质缓冲液相结合,以确定活性酶复合物 α 2 β 2中三个自由基中间体处有序水分子的存在大肠杆菌核糖核苷酸还原酶。我们的数据提供了明确的证据,表明所有三种单独捕获的中间体都与一个水分子超精细耦合,Tyr-O… 17 O 距离在 2.8–3.1 Å 范围内。该结构信息的可用性将允许在该原型酶中建立 PCET 的定量模型。结果还提供了与酪氨酰自由基键合的水的光谱特征。















































 京公网安备 11010802027423号
京公网安备 11010802027423号