当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Selective CO2 Electroreduction to C2H4 Using a Metal–Organic Framework with Dual Active Sites
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-05-06 , DOI: 10.1021/jacs.1c01466
Xiao-Feng Qiu 1 , Hao-Lin Zhu 1 , Jia-Run Huang 1 , Pei-Qin Liao 1 , Xiao-Ming Chen 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-05-06 , DOI: 10.1021/jacs.1c01466
Xiao-Feng Qiu 1 , Hao-Lin Zhu 1 , Jia-Run Huang 1 , Pei-Qin Liao 1 , Xiao-Ming Chen 1
Affiliation
|
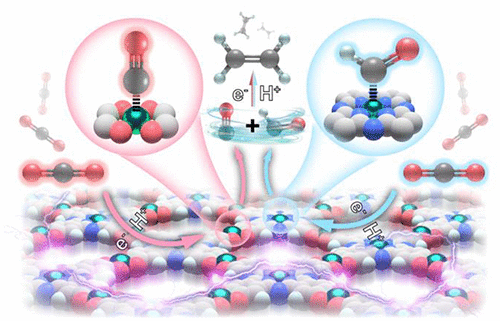
Conversion from CO2 to C2H4 is important for the development of energy and the environment, but the high energy barrier of hydrogenation of the *CO intermediate and C–C coupling step tend to result in C1 compounds as the main product and thus restrict the generation of C2H4. Here, we report a metal–organic framework (denoted as PcCu-Cu-O), composed of 2,3,9,10,16,17,23,24-octahydroxyphthalo-cyaninato)copper(II) (PcCu-(OH)8) ligands and the square-planar CuO4 nodes, as the electrocatalyst for CO2 to C2H4. Compared with the discrete molecular copper-phthalocyanine (Faradaic efficiency (FE) of C2H4 = 25%), PcCu-Cu-O exhibits much higher performance for electrocatalytic reduction of CO2 to C2H4 with a FE of 50(1)% and a current density of 7.3 mA cm–2 at the potential of −1.2 V vs RHE in 0.1 M KHCO3 solution, representing the best performance reported to date. In-situ infrared spectroscopy and control experiments suggested that the enhanced electrochemical performance may be ascribed to the synergistic effect between the CuPc unit and the CuO4 unit, namely the CO on the CO-producing site (CuO4 site) can efficiently migrate and dimerize with the *CO intermediate adsorbed on the C2H4-producing site (CuPc), giving a lower C–C dimerization energy barrier.
中文翻译:

使用具有双重活性位点的金属有机骨架,将高选择性的CO 2电还原为C 2 H 4
从CO 2到C 2 H 4的转化对于能源和环境的发展很重要,但是* CO中间体加氢和C–C偶联步骤的高能垒倾向于导致C 1化合物成为主要产物,因此限制了C 2 H 4的产生。在这里,我们报告一个金属-有机骨架(表示为PcCu-Cu-O),由2,3,9,10,16,17,23,24-八羟基邻苯二甲腈-氰基铜)(II)(PcCu-(OH )8)配体和方形的CuO 4结点,作为CO 2至C 2 H 4的电催化剂。与(C的法拉第效率(FE)的离散的分子铜酞菁相比2 ħ 4,= 25%)酞菁铜铜氧为电催化还原CO的性能表现出高得多2至C 2 ħ 4具有50 FE(在0.1 M KHCO 3溶液中,RHE为-1)%,相对于RHE的电位为–1.2 V时的电流密度为7.3 mA cm –2,代表了迄今为止报道的最佳性能。原位红外光谱和控制实验表明,增强的电化学性能可能归因于CuPc单元与CuO 4之间的协同作用单位,即CO产生位点(CuO 4位点)上的CO可以有效地迁移并与吸附在C 2 H 4产生位点(CuPc)上的* CO中间体二聚,从而提供较低的CC二聚能垒。
更新日期:2021-05-19
中文翻译:

使用具有双重活性位点的金属有机骨架,将高选择性的CO 2电还原为C 2 H 4
从CO 2到C 2 H 4的转化对于能源和环境的发展很重要,但是* CO中间体加氢和C–C偶联步骤的高能垒倾向于导致C 1化合物成为主要产物,因此限制了C 2 H 4的产生。在这里,我们报告一个金属-有机骨架(表示为PcCu-Cu-O),由2,3,9,10,16,17,23,24-八羟基邻苯二甲腈-氰基铜)(II)(PcCu-(OH )8)配体和方形的CuO 4结点,作为CO 2至C 2 H 4的电催化剂。与(C的法拉第效率(FE)的离散的分子铜酞菁相比2 ħ 4,= 25%)酞菁铜铜氧为电催化还原CO的性能表现出高得多2至C 2 ħ 4具有50 FE(在0.1 M KHCO 3溶液中,RHE为-1)%,相对于RHE的电位为–1.2 V时的电流密度为7.3 mA cm –2,代表了迄今为止报道的最佳性能。原位红外光谱和控制实验表明,增强的电化学性能可能归因于CuPc单元与CuO 4之间的协同作用单位,即CO产生位点(CuO 4位点)上的CO可以有效地迁移并与吸附在C 2 H 4产生位点(CuPc)上的* CO中间体二聚,从而提供较低的CC二聚能垒。

































 京公网安备 11010802027423号
京公网安备 11010802027423号