当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic Synthesis of Urea (CO2/N2/H2O) on Coal-Based Carbon Nanotubes with the Fe-Core-Supported Ti3+-TiO2 Composite Catalyst
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-05-07 , DOI: 10.1021/acssuschemeng.1c00644 Halidan Maimaiti 1 , Bo Xu 1 , Jin-yan Sun 1 , Li-rong Feng 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-05-07 , DOI: 10.1021/acssuschemeng.1c00644 Halidan Maimaiti 1 , Bo Xu 1 , Jin-yan Sun 1 , Li-rong Feng 1
Affiliation
|
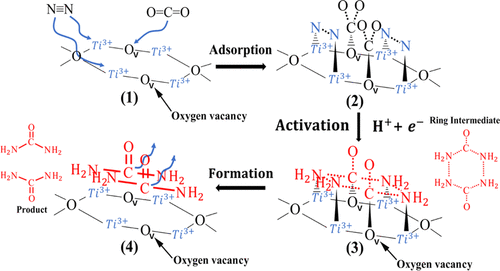
In this study, carbon nanotubes with Fe cores (Fe-CNTs) were successfully prepared by the vapor deposition method, which used raw coal as a carbon source. The brookite crystal Ti3+-doped TiO2 (Ti3+-TiO2) was obtained via thermal reduction with the reductant NaBH4. Then, it was loaded on Fe-CNTs to obtain Ti3+-TiO2/Fe-CNTs. While characterizing the structure of these prepared catalysts, the photocatalytic co-reduction of N2/CO2 was performed for the synthesis of urea (CO(NH2)2) in H2O. The findings were as follows: (i) the reductant NaBH4 could lead to the formation of the brookite crystal TiO2; (ii) the main product of the photocatalytic co-reduction N2/CO2 in H2O is CO(NH2)2, and it also includes the gas products (H2, CO, and O2) and other liquid products (NH4+, NO2–, NO3–, C3H6O2, and C4H8O2); (iii) the performance of photocatalytic co-reduction of N2/CO2 to CO(NH2)2 in H2O was related to the arrangement of Ti3+ sites and oxygen vacancies on the surface of Ti3+-TiO2. The Ti3+ sites and oxygen vacancies act as the active centers for N2 and CO2 molecules, respectively. The adsorption and activation converted the N2 and CO2 molecules into six-membered cyclic intermediates, which further transformed into the CO(NH2)2 product. In addition, the CO(NH2)2 yield of the composite photocatalyst can reach 710.1 μmol/(L g) in a 4 h reaction, with 4.6 times as the single Ti3+-TiO2, which shows that the Fe-CNT support is beneficial for the photocatalysis.
中文翻译:

铁芯负载的Ti 3+ -TiO 2复合催化剂在煤基碳纳米管上光催化合成尿素(CO 2 / N 2 / H 2 O)
本研究以原煤为碳源,通过气相沉积法成功制备了具有铁芯的碳纳米管(Fe-CNTs)。通过用还原剂NaBH 4进行热还原,获得了板钛矿型掺杂Ti 3+的TiO 2(Ti 3+ -TiO 2)。然后,将其负载在Fe-CNT上以获得Ti 3+ -TiO 2 / Fe-CNT。在表征这些制备的催化剂的结构的同时,进行了N 2 / CO 2的光催化共还原,以在H 2 O中合成尿素(CO(NH 2)2)。还原剂NaBH 4可能导致板钛矿晶体TiO 2的形成; (ii)H 2 O中的光催化共还原N 2 / CO 2的主要产物是CO(NH 2)2,它还包括气体产物(H 2,CO和O 2)和其他液体产物(NH 4+,NO 2 -,NO 3 -,C 3 H ^ 6 Ò 2,和C 4 H ^ 8 ö 2); (iii)N 2 / CO 2的光催化共还原性能H 2 O中CO(NH 2)2的含量与Ti 3+ -TiO 2表面上Ti 3+位点的排列和氧空位有关。Ti 3+位点和氧空位分别充当N 2和CO 2分子的活性中心。吸附和活化将N 2和CO 2分子转化为六元环状中间体,然后进一步转化为CO(NH 2)2产物。另外,CO(NH 2)2在4 h反应中,复合光催化剂的产率可达到710.1μmol/(L g),是单一Ti 3+ -TiO 2的4.6倍,表明Fe-CNT载体对光催化是有益的。
更新日期:2021-05-24
中文翻译:

铁芯负载的Ti 3+ -TiO 2复合催化剂在煤基碳纳米管上光催化合成尿素(CO 2 / N 2 / H 2 O)
本研究以原煤为碳源,通过气相沉积法成功制备了具有铁芯的碳纳米管(Fe-CNTs)。通过用还原剂NaBH 4进行热还原,获得了板钛矿型掺杂Ti 3+的TiO 2(Ti 3+ -TiO 2)。然后,将其负载在Fe-CNT上以获得Ti 3+ -TiO 2 / Fe-CNT。在表征这些制备的催化剂的结构的同时,进行了N 2 / CO 2的光催化共还原,以在H 2 O中合成尿素(CO(NH 2)2)。还原剂NaBH 4可能导致板钛矿晶体TiO 2的形成; (ii)H 2 O中的光催化共还原N 2 / CO 2的主要产物是CO(NH 2)2,它还包括气体产物(H 2,CO和O 2)和其他液体产物(NH 4+,NO 2 -,NO 3 -,C 3 H ^ 6 Ò 2,和C 4 H ^ 8 ö 2); (iii)N 2 / CO 2的光催化共还原性能H 2 O中CO(NH 2)2的含量与Ti 3+ -TiO 2表面上Ti 3+位点的排列和氧空位有关。Ti 3+位点和氧空位分别充当N 2和CO 2分子的活性中心。吸附和活化将N 2和CO 2分子转化为六元环状中间体,然后进一步转化为CO(NH 2)2产物。另外,CO(NH 2)2在4 h反应中,复合光催化剂的产率可达到710.1μmol/(L g),是单一Ti 3+ -TiO 2的4.6倍,表明Fe-CNT载体对光催化是有益的。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号