Steroids Pub Date : 2021-05-07 , DOI: 10.1016/j.steroids.2021.108858
Prima Francis 1 , Kajal Chakraborty 1
|
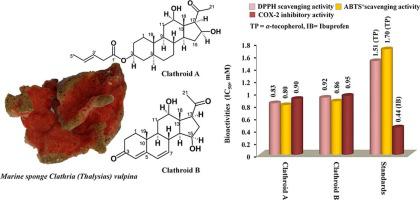
Two pregnane-type of steroid derivatives characterized as 5α-pregna-3β-methyl pent-3-enoate-12β, 16β diol-20-one (clathroid A) and 12β,15β- dihydroxypregna-4,6-diene-3,20-dione (clathroid B) were purified from the crude extract of the marine sponge, Clathria (Thalysias) vulpina (family Microcionidae) by extensive chromatographic fractionation. Spectroscopic methods including nuclear magnetic resonance spectroscopy were employed to characterize the purified clathroids A-B. The studied compounds exhibited duel inhibitory potentials against pro-inflammatory cyclooxygenase-2 and 5-lipoxygenase (median inhibitory concentration, IC50 < 1 mM), whereas the attenuation property of clathroid A against 5-lipoxygenase (IC50 0.85 mM) was greater than the standard anti-inflammatory ibuprofen (IC50 4.51 mM, p < 0.05). Greater selectivity index (anti cyclooxygense-2/anti cyclooxygense-1) of the studied clathroids (>1) than ibuprofen (0.43) attributed the greater selective attenuation properties towards pro-inflammatory inducible cyclooxygenase-2 than its constitutive isoenzyme cyclooxygenase-1. The antioxidant potentials of clathroid A against 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (IC50 0.80 mM) and diphenyl-1-picrylhydrazyl (IC50 0.83 mM) free radicals were greater than those of clathroid B (IC50 0.86–0.96 mM). Structure–activity analyses showed that the bioactivities of the clathroids were directly related to their electronic parameters coupled with permissible hydrophobic properties. Clathroid A exhibited grater electronic parameter (topological polar surface area tPSA, 83.83) than clathroid B (74.60) and ibuprofen (37.30), which were found to be in agreement with the prospective anti-inflammatory profile of clathroid A. Clathroid A exhibited higher number of hydrogen bonding interactions with 5-lipoxygenase active site and lesser docking values, such as docking score (DS −12.90 kcal mol−1) and inhibition constant (Ki 1.11 nM) than those recorded by clathroid B (DS −10.49 kcal mol−1; Ki 13.88 nM). The molecular binding properties of clathroid A with 5-lipoxidase inferred that its docking score/ binding energy were positively correlated with their in vitro bioactivie potentilas. A putative biosynthetic pathway of the studied clathroids was proposed from a pregnenolone precursor. The present study recognized the potential of clathroid A isolated from C. (Thalysias) vulpina as prospective anti-inflammatory lead that could find its use in medicinal applications.
中文翻译:

来自海洋 Microcionidae 海绵的抗炎孕烷类类固醇衍生物类固醇 AB
两种孕烷型类固醇衍生物,其特征为 5 α -pregna-3 β -methyl pent-3-enoate-12 β、16 β diol-20-one (clathroid A) 和 12 β ,15 β - dihydroxypregna-4,6 -diene-3,20-dione (clathroid B ) 通过广泛的色谱分离从海海绵Clathria (Thalysias) vulpina (Microcionidae) 的粗提物中纯化。包括核磁共振波谱在内的光谱方法用于表征纯化的类固醇 AB。研究的化合物对促炎性环氧合酶 2 和 5-脂氧合酶表现出双重抑制潜力(中位抑制浓度,IC 50 < 1 mM),而 clathroid A 对 5-脂氧合酶 (IC 50 0.85 mM) 的衰减特性大于标准抗炎布洛芬 (IC 50 4.51 mM, p < 0.05)。与布洛芬 (0.43) 相比,所研究的类固醇 (>1) 的选择性指数 (抗 cyclooxygense-2/抗 cyclooxygenase-1) 更大,这归因于促炎诱导型 cyclooxygenase-2 比其组成型同工酶 cyclooxygenase-1 具有更大的选择性衰减特性。clathroid A 对 2, 2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (IC 50 0.80 mM) 和 diphenyl-1-picrylhydrazyl (IC 50 0.83 mM) 自由基的抗氧化能力高于类甲状腺素 B (IC 500.86–0.96 毫米)。结构-活性分析表明,包合物的生物活性与其电子参数以及允许的疏水特性直接相关。网格 A 表现出比网格 B (74.60) 和布洛芬 (37.30) 更高的电子参数 (拓扑极性表面积 tPSA, 83.83),这与网格 A 的预期抗炎特性一致。网格 A 表现出更高的数量与 5-脂氧合酶活性位点的氢键相互作用和较小的对接值,例如对接分数 (DS -12.90 kcal mol -1 ) 和抑制常数 (K i 1.11 nM) 比通过类固醇 B (DS -10.49 kcal mol - 1 ; K我13.88 纳米)。clathroid A与5-脂氧化酶的分子结合特性推断其对接分数/结合能与其体外生物活性电位呈正相关。从孕烯醇酮前体提出了所研究的类固醇的推定生物合成途径。本研究认识到从C. (Thalysias) vulpina中分离的 clathroid A作为潜在的抗炎先导剂的潜力,可在药物应用中找到其用途。





































 京公网安备 11010802027423号
京公网安备 11010802027423号