当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Atroposelective Rhodium(II)-Catalyzed N–H Bond Insertion: Access to Axially Chiral N-Arylindolocarbazoles
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-05-06 , DOI: 10.1021/acscatal.1c01232 Qiao Ren 1, 2 , Tingting Cao 1 , Chunnian He 1 , Meihua Yang 1 , Haitao Liu 1 , Lei Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-05-06 , DOI: 10.1021/acscatal.1c01232 Qiao Ren 1, 2 , Tingting Cao 1 , Chunnian He 1 , Meihua Yang 1 , Haitao Liu 1 , Lei Wang 1
Affiliation
|
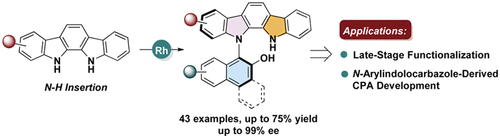
The highly atroposelective construction of axially chiral N-arylindolocarbazoles is disclosed via the Rh(II)-catalyzed intermolecular carbene N–H insertion reactions. This straightforward transformation provides a rapid access to a wide range of enantio-enriched N-arylindolocarbazole atropisomers bearing the C–N axis in moderate to good yields and high enantioselectivities. Also, the synthetic utility of this protocol is unambiguously emphasized by the late-stage functionalization (LSF) of some representative natural products and bioactive molecules. Furthermore, the polyaromatic ring construction and N-arylindolocarbazole-derived chiral phosphoric acid (CPA) synthesis also highlight its practical value in materials chemistry and catalytic asymmetric synthesis.
中文翻译:

高度对映选择性的铑(II)催化的NH键插入:获得轴向手性N-芳基吲哚并咔唑
通过Rh(II)催化的分子间卡宾NH插入反应公开了轴向手性N-芳基吲哚并咔唑的高度对映选择性。这种简单的转化可以快速获得各种带有C–N轴的对映体富集的N-芳基吲哚并咔唑阻转异构体,且产率中等至良好,对映选择性很高。同样,某些代表性天然产物和生物活性分子的后期功能化(LSF)明确强调了该协议的合成效用。此外,聚芳环的结构和N芳基吲哚并咔唑衍生的手性磷酸(CPA)合成也突出了其在材料化学和催化不对称合成中的实用价值。
更新日期:2021-05-22
中文翻译:

高度对映选择性的铑(II)催化的NH键插入:获得轴向手性N-芳基吲哚并咔唑
通过Rh(II)催化的分子间卡宾NH插入反应公开了轴向手性N-芳基吲哚并咔唑的高度对映选择性。这种简单的转化可以快速获得各种带有C–N轴的对映体富集的N-芳基吲哚并咔唑阻转异构体,且产率中等至良好,对映选择性很高。同样,某些代表性天然产物和生物活性分子的后期功能化(LSF)明确强调了该协议的合成效用。此外,聚芳环的结构和N芳基吲哚并咔唑衍生的手性磷酸(CPA)合成也突出了其在材料化学和催化不对称合成中的实用价值。































 京公网安备 11010802027423号
京公网安备 11010802027423号