Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2021-05-06 , DOI: 10.1016/j.jclepro.2021.127384
Xiuling Guo , Yang Ruan , Zenghui Diao , Kaimin Shih , Minhua Su , Gang Song , Diyun Chen , Shuao Wang , Lingjun Kong
|
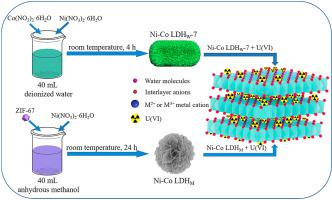
With the wide application of nuclear energy and the rapid development of mining, a great quantity of uranium (U(VI)) containing wastewater is inevitably generated. In this work, a facile environmental-friendly coprecipitation method was proposed for the green synthesis of binary layered double hydroxides (Ni–Co LDHW-7), which could be used to efficiently adsorb U(VI) from wastewater. Pseudo-second-order kinetic model and Dubinin-Radushkevich model fitted well with the adsorption of U(VI) on Ni–Co LDHW-7, the maximum adsorption capacity of U(VI) on Ni–Co LDHW-7 was 201.09 mg/g calculated by the Langmuir model. Adsorption equilibrium reached after 30 min, which is a spontaneous and endothermic process. Fourier Transform Infrared Spectrometer (FTIR) and X-ray Photoelectron Spectroscopy (XPS) measurements manifested that the favorable U(VI) extraction on Ni–Co LDHW-7 is mainly ascribed to inner-layer surface electrostatic interaction and complexation, which is dominated by abundant oxygen-containing M-OH and interlayer OH− ions. Briefly, this work provides a facial and environmental-friendly Ni–Co LDHW-7 for removing U(VI) in actual uranium-containing wastewater.
中文翻译:

Ni-Co层状双氢氧化物(LDH)分层纳米阵列的环保制备,可有效去除铀(VI)
随着核能的广泛应用和采矿业的迅猛发展,不可避免地会产生大量的含铀废水。在这项工作中,为绿色合成二元层状双氢氧化物(Ni-Co LDH W -7)提出了一种简便的环保共沉淀方法,该方法可用于有效地吸附废水中的U(VI)。伪二级动力学模型和杜比宁-Radushkevich模型拟合以及带U的上镍钴LDH吸附(VI)w ^上镍钴LDH -7,U的最大吸附容量(VI)w ^-7是通过Langmuir模型计算得出的201.09 mg / g。30分钟后达到吸附平衡,这是一个自发的吸热过程。傅立叶变换红外光谱仪(FTIR)和X射线光电子能谱仪(XPS)测量表明,对Ni-Co LDH W -7进行有利的U(VI)萃取主要归因于内层表面静电相互作用和络合作用,这是主要的原因。通过丰富M-OH和层间OH含氧-离子。简而言之,这项工作为去除实际含铀废水中的U(VI)提供了一种面部和环境友好的Ni-Co LDH W -7。

































 京公网安备 11010802027423号
京公网安备 11010802027423号