当前位置:
X-MOL 学术
›
J. Neurosci. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Endogenous µ-opioid receptor activity in the lateral and capsular subdivisions of the right central nucleus of the amygdala prevents chronic postoperative pain
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2021-05-06 , DOI: 10.1002/jnr.24846 Andrew H Cooper 1 , Naomi S Hedden 2 , Gregory Corder 3, 4 , Sydney R Lamerand 2, 5 , Renee R Donahue 6 , Julio C Morales-Medina 6 , Lindsay Selan 2 , Pranav Prasoon 2 , Bradley K Taylor 2
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2021-05-06 , DOI: 10.1002/jnr.24846 Andrew H Cooper 1 , Naomi S Hedden 2 , Gregory Corder 3, 4 , Sydney R Lamerand 2, 5 , Renee R Donahue 6 , Julio C Morales-Medina 6 , Lindsay Selan 2 , Pranav Prasoon 2 , Bradley K Taylor 2
Affiliation
|
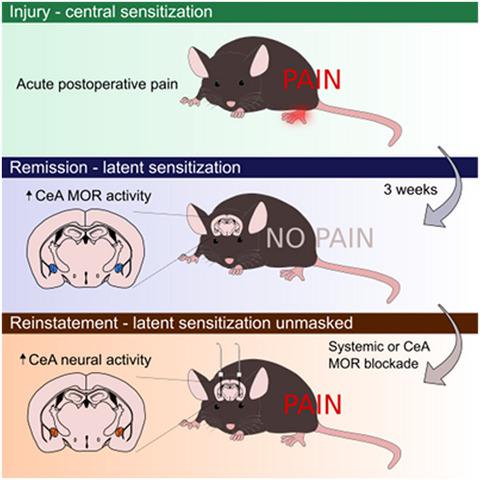
Tissue injury induces a long-lasting latent sensitization (LS) of spinal nociceptive signaling that is kept in remission by an opposing µ-opioid receptor (MOR) constitutive activity. To test the hypothesis that supraspinal sites become engaged, we induced hindpaw inflammation, waited 3 weeks for mechanical hypersensitivity to resolve, and then injected the opioid receptor inhibitors naltrexone, CTOP or β-funaltrexamine subcutaneously, and/or into the cerebral ventricles. Intracerebroventricular injection of each inhibitor reinstated hypersensitivity and produced somatic signs of withdrawal, indicative of LS and endogenous opioid dependence, respectively. In naïve or sham controls, systemic naloxone (3 mg/kg) produced conditioned place aversion, and systemic naltrexone (3 mg/kg) increased Fos expression in the central nucleus of the amygdala (CeA). In LS animals tested 3 weeks after plantar incision, systemic naltrexone reinstated mechanical hypersensitivity and produced an even greater increase in Fos than in sham controls, particularly in the capsular subdivision of the right CeA. One third of Fos+ profiles co-expressed protein kinase C delta (PKCδ), and 35% of PKCδ neurons co-expressed tdTomato+ in Oprm1Cre::tdTomato transgenic mice. CeA microinjection of naltrexone (1 µg) reinstated mechanical hypersensitivity only in male mice and did not produce signs of somatic withdrawal. Intra-CeA injection of the MOR-selective inhibitor CTAP (300 ng) reinstated hypersensitivity in both male and female mice. We conclude that MORs in the capsular subdivision of the right CeA prevent the transition from acute to chronic postoperative pain.
中文翻译:

杏仁核右侧中央核外侧和囊膜细分中的内源性 μ-阿片受体活性可防止慢性术后疼痛
组织损伤诱导脊髓伤害性信号传导的长期潜伏敏化 (LS),该信号传导被相反的 μ-阿片受体 (MOR) 组成型活性保持缓解。为了检验脊髓上部位参与的假设,我们诱导了后爪炎症,等待 3 周让机械超敏反应消退,然后皮下注射阿片受体抑制剂纳曲酮、CTOP 或 β-funaltrexamine 和/或进入脑室。每种抑制剂的脑室内注射可恢复超敏反应并产生戒断的躯体体征,分别表明 LS 和内源性阿片类药物依赖。在初始或假对照中,全身性纳洛酮 (3 mg/kg) 产生条件性地方厌恶,全身性纳曲酮 (3 mg/kg) 增加杏仁核 (CeA) 中 Fos 的表达。在足底切口后 3 周测试的 LS 动物中,全身性纳曲酮恢复了机械超敏反应,并且产生的 Fos 增加甚至比假对照更大,特别是在右侧 CeA 的包膜细分中。在 Oprm1Cre::tdTomato 转基因小鼠中,1/3 的 Fos+ 谱共表达蛋白激酶 C δ (PKCδ),35% 的 PKCδ 神经元共表达 tdTomato+。纳曲酮 (1 μg) 的 CeA 显微注射仅在雄性小鼠中恢复机械超敏反应,并且没有产生躯体戒断迹象。在 CEA 内注射 MOR 选择性抑制剂 CTAP (300 ng) 可恢复雄性和雌性小鼠的超敏反应。我们得出结论,右侧 CeA 囊细分中的 MORs 可防止术后疼痛从急性向慢性的转变。
更新日期:2021-05-06
中文翻译:

杏仁核右侧中央核外侧和囊膜细分中的内源性 μ-阿片受体活性可防止慢性术后疼痛
组织损伤诱导脊髓伤害性信号传导的长期潜伏敏化 (LS),该信号传导被相反的 μ-阿片受体 (MOR) 组成型活性保持缓解。为了检验脊髓上部位参与的假设,我们诱导了后爪炎症,等待 3 周让机械超敏反应消退,然后皮下注射阿片受体抑制剂纳曲酮、CTOP 或 β-funaltrexamine 和/或进入脑室。每种抑制剂的脑室内注射可恢复超敏反应并产生戒断的躯体体征,分别表明 LS 和内源性阿片类药物依赖。在初始或假对照中,全身性纳洛酮 (3 mg/kg) 产生条件性地方厌恶,全身性纳曲酮 (3 mg/kg) 增加杏仁核 (CeA) 中 Fos 的表达。在足底切口后 3 周测试的 LS 动物中,全身性纳曲酮恢复了机械超敏反应,并且产生的 Fos 增加甚至比假对照更大,特别是在右侧 CeA 的包膜细分中。在 Oprm1Cre::tdTomato 转基因小鼠中,1/3 的 Fos+ 谱共表达蛋白激酶 C δ (PKCδ),35% 的 PKCδ 神经元共表达 tdTomato+。纳曲酮 (1 μg) 的 CeA 显微注射仅在雄性小鼠中恢复机械超敏反应,并且没有产生躯体戒断迹象。在 CEA 内注射 MOR 选择性抑制剂 CTAP (300 ng) 可恢复雄性和雌性小鼠的超敏反应。我们得出结论,右侧 CeA 囊细分中的 MORs 可防止术后疼痛从急性向慢性的转变。































 京公网安备 11010802027423号
京公网安备 11010802027423号