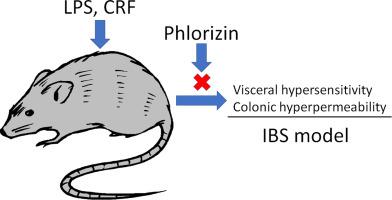
内脏超敏反应和肠壁屏障受损是肠易激综合征(IBS)病理生理的关键因素,而这些经由糖皮质激素释放因子(CRF)-Toll受体4-促炎性细胞因子信号传导介导。Phlorizin是钠连接的葡萄糖转运蛋白(SGLTs)的抑制剂,已知具有抗细胞因子特性。因此,我们假设phlorizin可能会改善IBS的这些胃肠道变化,并在大鼠IBS模型即脂多糖(LPS)或CRF引起的内脏超敏和结肠通透性过高的情况下验证了这一假设。通过肌电图通过腹部肌肉收缩来估计响应结肠球囊扩张的内脏疼痛阈值,并且通过量化在结肠组织中吸收的伊文思蓝来测量结肠通透性。皮下(sc phlorizin注射以剂量依赖性方式抑制LPS诱导的内脏超敏性和结肠通透性。Phlorizin还阻止了CRF诱导的这些胃肠道改变。众所周知,Phlorizin可以抑制SGLT1和SGLT2,但是胃内施用Phlorizin只能抑制SGLT1,因为肠道主要表达SGLT1。我们发现胃内phlorizin并未显示任何作用,但是口服活性且选择性的SGLT2抑制剂ipragliflozin改善了LPS模型中的胃肠道变化。化合物C,一磷酸腺苷激活的蛋白激酶(AMPK)抑制剂,N 众所周知,Phlorizin可以抑制SGLT1和SGLT2,但是胃内施用Phlorizin只能抑制SGLT1,因为肠道主要表达SGLT1。我们发现胃内phlorizin并未显示任何作用,但是口服活性且选择性的SGLT2抑制剂ipragliflozin改善了LPS模型中的胃肠道变化。化合物C,一磷酸腺苷激活的蛋白激酶(AMPK)抑制剂,N 众所周知,Phlorizin可以抑制SGLT1和SGLT2,但是胃内施用Phlorizin只能抑制SGLT1,因为肠道主要表达SGLT1。我们发现胃内phlorizin并未显示任何作用,但是口服活性且选择性的SGLT2抑制剂ipragliflozin改善了LPS模型中的胃肠道变化。化合物C,一磷酸腺苷激活的蛋白激酶(AMPK)抑制剂,N一氧化氮(NO)合成抑制剂G-硝基-L-精氨酸甲酯和阿片受体拮抗剂纳洛酮逆转了phlorizin的作用。总之,phlorizin可改善IBS模型中的内脏超敏性和结肠高通透性。这些作用可能是由于对SGLT2的抑制所致,并且是通过AMPK,NO和阿片类药物途径介导的。Phrizrizin可能对IBS的治疗有效。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Phlorizin attenuates visceral hypersensitivity and colonic hyperpermeability in a rat model of irritable bowel syndrome
Visceral hypersensitivity and impaired gut barrier are crucial contributors to the pathophysiology of irritable bowel syndrome (IBS), and those are mediated via corticotropin-releasing factor (CRF)−Toll like receptor 4–pro-inflammatory cytokine signaling. Phlorizin is an inhibitor of sodium-linked glucose transporters (SGLTs), and known to have anti-cytokine properties. Thus, we hypothesized that phlorizin may improve these gastrointestinal changes in IBS, and tested this hypothesis in rat IBS models, i.e., lipopolysaccharide (LPS) or CRF-induced visceral hypersensitivity and colonic hyperpermeability. The visceral pain threshold in response to colonic balloon distention was estimated by abdominal muscle contractions by electromyogram, and colonic permeability was measured by quantifying the absorbed Evans blue in colonic tissue. Subcutaneous (s.c.) injection of phlorizin inhibited visceral hypersensitivity and colonic hyperpermeability induced by LPS in a dose-dependent manner. Phlorizin also blocked CRF-induced these gastrointestinal changes. Phlorizin is known to inhibit both SGLT1 and SGLT2, but intragastric administration of phlorizin may only inhibit SGLT1 because gut mainly expresses SGLT1. We found that intragastric phlorizin did not display any effects, but ipragliflozin, an orally active and selective SGLT2 inhibitor improved the gastrointestinal changes in the LPS model. Compound C, an adenosine monophosphate-activated protein kinase (AMPK) inhibitor, NG-nitro-L-arginine methyl ester, a nitric oxide (NO) synthesis inhibitor and naloxone, an opioid receptor antagonist reversed the effects of phlorizin. In conclusions, phlorizin improved visceral hypersensitivity and colonic hyperpermeability in IBS models. These effects may result from inhibition of SGLT2, and were mediated via AMPK, NO and opioid pathways. Phlorizin may be effective for the treatment of IBS.
































 京公网安备 11010802027423号
京公网安备 11010802027423号