当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promiscuous Esterases Counterintuitively Are Less Flexible than Specific Ones
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-05-05 , DOI: 10.1021/acs.jcim.1c00152 Christina Nutschel 1 , Cristina Coscolín 2 , Benoit David 3 , Daniel Mulnaes 3 , Manuel Ferrer 2 , Karl-Erich Jaeger 4, 5 , Holger Gohlke 1, 3
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2021-05-05 , DOI: 10.1021/acs.jcim.1c00152 Christina Nutschel 1 , Cristina Coscolín 2 , Benoit David 3 , Daniel Mulnaes 3 , Manuel Ferrer 2 , Karl-Erich Jaeger 4, 5 , Holger Gohlke 1, 3
Affiliation
|
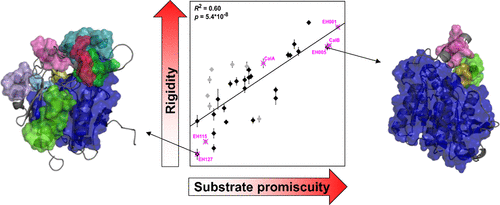
Understanding mechanisms of promiscuity is increasingly important from a fundamental and application point of view. As to enzyme structural dynamics, more promiscuous enzymes generally have been recognized to also be more flexible. However, examples for the opposite received much less attention. Here, we exploit comprehensive experimental information on the substrate promiscuity of 147 esterases tested against 96 esters together with computationally efficient rigidity analyses to understand the molecular origin of the observed promiscuity range. Unexpectedly, our data reveal that promiscuous esterases are significantly less flexible than specific ones, are significantly more thermostable, and have a significantly increased specific activity. These results may be reconciled with a model according to which structural flexibility in the case of specific esterases serves for conformational proofreading. Our results signify that an esterase sequence space can be screened by rigidity analyses for promiscuous esterases as starting points for further exploration in biotechnology and synthetic chemistry.
中文翻译:

直觉上混杂的酯酶比特定的酶缺乏弹性
从基础和应用的角度来看,了解滥交机制变得越来越重要。关于酶的结构动力学,一般认为混杂的酶也更灵活。然而,相反的例子却很少受到关注。在这里,我们利用针对96种酯测试的147种酯酶的底物混杂度综合了实验信息,并通过有效的刚性分析来了解所观察到的混杂度范围的分子起源。出乎意料的是,我们的数据表明,混杂酯酶的柔韧性明显低于特定酯酶,热稳定性明显更高,并且比活性显着提高。这些结果可以与一个模型相一致,根据该模型,在特定酯酶的情况下,结构灵活性可用于构象校对。我们的结果表明,可以通过对混杂酯酶进行刚性分析来筛选酯酶序列空间,以此作为在生物技术和合成化学中进一步探索的起点。
更新日期:2021-05-24
中文翻译:

直觉上混杂的酯酶比特定的酶缺乏弹性
从基础和应用的角度来看,了解滥交机制变得越来越重要。关于酶的结构动力学,一般认为混杂的酶也更灵活。然而,相反的例子却很少受到关注。在这里,我们利用针对96种酯测试的147种酯酶的底物混杂度综合了实验信息,并通过有效的刚性分析来了解所观察到的混杂度范围的分子起源。出乎意料的是,我们的数据表明,混杂酯酶的柔韧性明显低于特定酯酶,热稳定性明显更高,并且比活性显着提高。这些结果可以与一个模型相一致,根据该模型,在特定酯酶的情况下,结构灵活性可用于构象校对。我们的结果表明,可以通过对混杂酯酶进行刚性分析来筛选酯酶序列空间,以此作为在生物技术和合成化学中进一步探索的起点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号