International Journal of Pharmaceutics ( IF 5.3 ) Pub Date : 2021-05-04 , DOI: 10.1016/j.ijpharm.2021.120650 Yuanyuan Chen 1 , Die Jia 2 , Qiming Wang 1 , Yueru Sun 1 , Zhenan Rao 1 , Xiaojuan Lei 1 , Jichun Zhao 1 , Kaifang Zeng 3 , Zhigang Xu 2 , Jian Ming 3
|
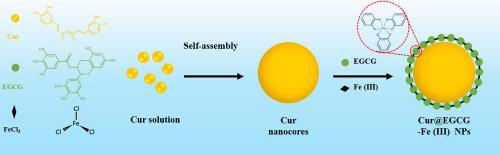
Curcumin (Cur), a hydrophobic active pharmaceutical ingredient with high anticancer activity, has poor water solubility and low bioavailability. Although many delivery systems have been developed to improve their bioavailability, some limitation such as low drug loading efficiency and poor stability are still remained. The metal-polyphenol networks (MPNs) delivery system designed in this subject solved above problems and effectively improved the anticancer activity of Cur. The synthesized Cur@EGCG-Fe(III) is consisting of epigallocatechin gallate (EGCG), iron chloride (FeCl3) and Cur, and the well-designed structure endow Cur@EGCG-Fe(III) high loading efficiency, good water solubility and stability. After the Cur@EGCG-Fe(III) nanoparticles were internalized by MCF-7 cells, the Cur could be released in endo/lysosomal microenvironment (pH = 5.0), and the Cur delivery in the deep tumor could be realized. The distribution of Cur@EGCG-Fe(III) in MCF-7 cells was analyzed by laser confocal, and Cur@EGCG-Fe(III) could effectively deliver more Cur into MCF-7 cells in comparison with free Cur. In addition, the results of flow cytometry and western blot further indicated that Cur@EGCG-Fe(III) had a stronger ability to induce apoptosis than free Cur. Transwell cell migration and invasion experiments showed that Cur and EGCG-Fe(III) had a synergistic effect in inhibiting MCF-7 cell migration and invasion. In vitro hemolysis and in vivo experiments showed that the Cur@EGCG-Fe(III) had negligible effect on the blood environment and a great tumor-inhibition efficacy, indicating that the MPNs delivery system had a good blood compatibility and antitumor activity. Our results indicated that MPNs-coated Cur nanoparticle could be a new form of Cur delivery system for anticancer application.
中文翻译:

基于金属-多酚网络传递系统促进姜黄素的抗癌活性
姜黄素(Cur)是一种具有高抗癌活性的疏水活性药物成分,水溶性差,生物利用度低。尽管已经开发了许多递送系统来改善其生物利用度,但是仍然存在一些局限性,例如低药物装载效率和较差的稳定性。本课题设计的金属多酚网络(MPNs)传递系统解决了上述问题,有效提高了Cur的抗癌活性。合成的Cur @ EGCG-Fe(III)由表没食子儿茶素没食子酸酯(EGCG),氯化铁(FeCl 3)和Cur,精心设计的结构赋予Cur @ EGCG-Fe(III)高负载效率,良好的水溶性和稳定性。通过MCF-7细胞将Cur @ EGCG-Fe(III)纳米粒子内在化后,Cur可以在内/溶酶体微环境(pH = 5.0)中释放,并可以实现Cur在深部肿瘤中的递送。通过激光共聚焦分析了Cur @ EGCG-Fe(III)在MCF-7细胞中的分布,与游离Cur相比,Cur @ EGCG-Fe(III)可以有效地向MCF-7细胞中递送更多的Cur。此外,流式细胞术和蛋白质印迹的结果进一步表明,Cur @ EGCG-Fe(III)诱导细胞凋亡的能力比游离Cur强。Transwell细胞迁移和侵袭实验表明,Cur和EGCG-Fe(III)在抑制MCF-7细胞迁移和侵袭中具有协同作用。体外溶血和体内实验表明,Cur @ EGCG-Fe(III)对血液环境的影响可忽略不计,并且具有很强的抑瘤功效,表明MPNs递送系统具有良好的血液相容性和抗肿瘤活性。我们的结果表明,MPNs包覆的Cur纳米粒子可能是抗癌应用中Cur传递系统的一种新形式。






























 京公网安备 11010802027423号
京公网安备 11010802027423号