Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2021-05-04 , DOI: 10.1016/j.freeradbiomed.2021.04.035 Zhu-Ying Yan 1 , Jing Chen 1 , Jie Shao 1 , Ze-Qing Jiao 2 , Tian-Shu Tang 1 , Miao Tang 1 , Zhi-Guo Sheng 1 , Li Mao 1 , Rong Huang 3 , Chun-Hua Huang 1 , Zhi-Hui Zhang 4 , Hong-Mei Su 2 , Ben-Zhan Zhu 5
|
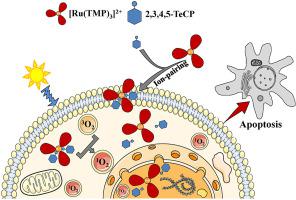
Developing the cell-impermeable Ru(II) polypyridyl cationic complexes as effective photosensitizers (PS) which have high cellular uptake and photo-toxicity, but low dark toxicity, is quite challenging. Here we found that the highly reactive singlet oxygen (1O2) can be generated by the irradiation of a typical Ru(II) polypyridyl complex Ru(II)tris(tetramethylphenanthroline) ([Ru(TMP)3]2+) under visible light irradiation by ESR with TEMPO (2,2,6,6-tetramethyl-4-piperidone-N-oxyl) as 1O2 probe. Effective cellular and nuclear delivery of cationic [Ru(TMP)3]2+ was achieved through our recently developed ion-pairing method, and 2,3,4,5-tetrachlorophenol (2,3,4,5-TeCP) was found to be the most effective among all chlorophenols tested. The accelerated cellular, especially nuclear uptake of [Ru(TMP)3]2+ results in the formation of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and DNA strand breaks, caspase 3/7 activation and cell apoptosis in HeLa cells upon light irradiation. More importantly, compared with other traditional photosensitizers, [Ru(TMP)3]2+ showed significant photo-toxicity but low dark toxicity. Similar effects were observed when 2,3,4,5-TeCP was substituted by the currently clinically used anti-inflammatory drug flufenamic acid. This represents the first report that the cell-impermeable Ru(II) polypyridyl complex ion-paired with suitable lipophilic counter-anions functions as potent intracellular photosensitizer under visible light irradiation mainly via a 1O2-mediated mechanism. These findings should provide new perspectives for future investigations on other metal complexes with similar characteristics as promising photosensitizers for potential photodynamic therapy.
中文翻译:

不可渗透的Ru(II)聚吡啶基络合物作为有效的细胞内光敏剂,在可见光照射下通过与合适的亲脂性抗衡离子进行离子配对
开发不透细胞的Ru(II)聚吡啶基阳离子复合物作为有效的光敏剂(PS),具有很高的细胞摄取量和光毒性,但暗毒性低,这是非常具有挑战性的。在这里,我们发现高活性单线态氧(1 O 2)可以通过在可见光下辐照典型的Ru(II)聚吡啶基络合物Ru(II)tris(tetramethylphenanthroline)([Ru(TMP)3 ] 2+)来产生。 ESR用TEMPO(2,2,6,6-四甲基-4-哌啶酮-N-氧基)作为1 O 2探针进行光照射。阳离子[Ru(TMP)3 ] 2+的有效细胞和核传递通过我们最近开发的离子对方法实现了这一目标,发现2,3,4,5-四氯苯酚(2,3,4,5-TeCP)在所有测试的氯酚中是最有效的。[Ru(TMP)3 ] 2+的细胞加速吸收,尤其是细胞核吸收,导致形成8-oxo-7,8-dihydro-2'-deoxyguanosine(8-oxodG)和DNA链断裂,胱天蛋白酶3/7照射后HeLa细胞中的活化和细胞凋亡。更重要的是,与其他传统的光敏剂相比,[Ru(TMP)3 ] 2+表现出显着的光毒性,但低暗毒性。当2,3,4,5-TeCP被目前临床上使用的消炎药氟芬那酸替代时,观察到类似的效果。这表示第一次报告该小区不透过性的Ru(II)多吡啶配合物离子对主要与合适的亲脂抗衡阴离子用作可见光照射下有效的细胞内光敏剂通过一个1 Ò 2介导的机制。这些发现应为将来进行其他金属配合物的研究提供新的视角,这些金属配合物具有与有望用于光动力疗法的光敏剂相似的特性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号