Chemical Physics Letters ( IF 2.8 ) Pub Date : 2021-05-03 , DOI: 10.1016/j.cplett.2021.138709 S.Vijayakumar , David M. Wilmouth
|
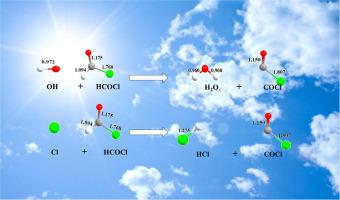
Formyl chloride (HCOCl) is a major product in the atmospheric degradation of several chlorinated hydrocarbons, but its atmospheric fate is not well known. Here, the kinetics of HCOCl with OH radicals and Cl atoms were studied over the temperature range of 180 to 400 K using Canonical Variational Transition state theory including Small Curvature Tunneling correction (CVT/SCT). The obtained temperature-dependent rate coefficients for the reactions of HCOCl with OH and Cl are (1.86 ± 0.7) × 10−11 exp[(−1217 ± 19)/T] cm3molecule−1s−1 and (9.87 ± 0.6) × 10−12 exp[(−747 ± 33)/T] cm3molecule−1s−1, respectively. Reaction mechanisms and thermodynamic parameters for the title reactions were studied, and the atmospheric lifetime of HCOCl was determined.
中文翻译:

甲酰氯的大气归宿及其与OH自由基和Cl原子的气相反应机理
甲酰氯(HCOCl)是几种氯代烃在大气中降解的主要产物,但其大气命运尚不为人所知。在此,使用包括小曲率隧穿校正(CVT / SCT)的规范变分过渡态理论,研究了在180至400 K的温度范围内具有OH自由基和Cl原子的HCOCl的动力学。所获得的HCOCl与OH和Cl反应的温度相关速率系数为(1.86±0.7)×10 -11 exp [(-1217±19)/ T] cm 3分子-1 s -1和(9.87±0.6 )×10 -12 exp [(-747±33)/ T] cm 3分子-1 s -1, 分别。研究了标题反应的反应机理和热力学参数,并确定了HCl的大气寿命。














































 京公网安备 11010802027423号
京公网安备 11010802027423号