Molecular Therapy: Oncology ( IF 5.3 ) Pub Date : 2021-04-29 , DOI: 10.1016/j.omto.2021.04.013 Shuyong Zhang 1, 2 , Dongdong Zhou 1 , Chao Zheng 1 , Peng Xiong 1 , Wan Zhu 1 , Dexian Zheng 1, 2
|
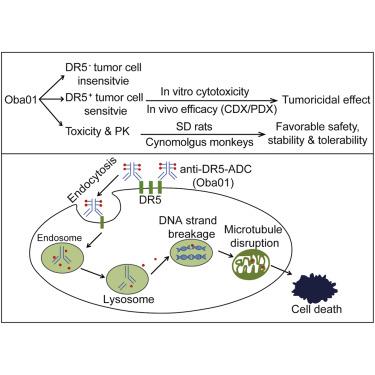
Acute lymphoblastic leukemia (ALL) is an aggressive hematological neoplasm resulting from immature lymphoid precursors. An antibody-drug conjugate (ADC), coupling a small molecule covalently with a targeting antibody, can specifically kill tumor cells. Death receptor 5 (DR5) is considered as a promising anti-tumor drug target. In this study, we describe the preclinical evaluation of a novel DR5-targeting ADC (Oba01) as a potential therapeutic against ALL. Oba01 utilizes anti-DR5 humanized monoclonal antibody (zaptuzumab) coupled via a cleavable linker to monomethyl auristatin E (MMAE). Oba01 can specifically bind to DR5 on the tumor cells and transfer into lysosome via DR5-mediated endocytosis. It then effectively releases the MMAE, which can bind to the tubulin and prevent its aggregation, thereby leading to a significant inhibition of proliferation and cell death in tumor cells. Additionally, Oba01 displays significant dose-dependent tumoricidal activity in cell-derived xenograft (CDX) and patient-derived xenograft (PDX) mouse models. More importantly, toxicity analysis of Oba01 showed a favorable safety profile, and pharmacokinetic analysis illustrated an excellent stability and tolerability in rats and cynomolgus monkeys. Taken together, our data conclusively demonstrate that Oba01 is an attractive candidate for further clinical trials in DR5-positive ALL patients.
中文翻译:

靶向 DR5 的新型抗体-药物偶联物用于淋巴细胞白血病治疗的临床前评估
急性淋巴细胞白血病 (ALL) 是一种由未成熟淋巴前体引起的侵袭性血液肿瘤。抗体药物偶联物 (ADC) 将小分子与靶向抗体共价偶联,可以特异性杀死肿瘤细胞。死亡受体 5 (DR5) 被认为是一种有前景的抗肿瘤药物靶点。在这项研究中,我们描述了一种新型 DR5 靶向 ADC (Oba01) 作为一种潜在的 ALL 治疗方法的临床前评估。Oba01 利用抗 DR5 人源化单克隆抗体 (zaptuzumab),通过可切割接头与单甲基 auristatin E (MMAE) 偶联。Oba01 可以与肿瘤细胞上的 DR5 特异性结合,并通过 DR5 介导的内吞作用转移到溶酶体中。然后它有效地释放 MMAE,它可以与微管蛋白结合并防止其聚集,从而导致显着抑制肿瘤细胞的增殖和细胞死亡。此外,Oba01 在细胞来源的异种移植物 (CDX) 和患者来源的异种移植物 (PDX) 小鼠模型中显示出显着的剂量依赖性杀肿瘤活性。更重要的是,Oba01 的毒性分析显示出良好的安全性,药代动力学分析表明在大鼠和食蟹猴中具有出色的稳定性和耐受性。总之,我们的数据最终证明 Oba01 是 DR5 阳性 ALL 患者进一步临床试验的有吸引力的候选者。Oba01 的毒性分析显示出良好的安全性,药代动力学分析表明在大鼠和食蟹猴中具有出色的稳定性和耐受性。总之,我们的数据最终证明 Oba01 是 DR5 阳性 ALL 患者进一步临床试验的有吸引力的候选者。Oba01 的毒性分析显示出良好的安全性,药代动力学分析表明在大鼠和食蟹猴中具有出色的稳定性和耐受性。总之,我们的数据最终证明 Oba01 是 DR5 阳性 ALL 患者进一步临床试验的有吸引力的候选者。















































 京公网安备 11010802027423号
京公网安备 11010802027423号