当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Counterion Binding Dynamics of a Polyelectrolyte
Macromolecules ( IF 5.1 ) Pub Date : 2021-04-27 , DOI: 10.1021/acs.macromol.1c00154
Yu Shi 1, 2 , Hao Peng 1, 2 , Jingfa Yang 1, 2 , Jiang Zhao 1, 2
Macromolecules ( IF 5.1 ) Pub Date : 2021-04-27 , DOI: 10.1021/acs.macromol.1c00154
Yu Shi 1, 2 , Hao Peng 1, 2 , Jingfa Yang 1, 2 , Jiang Zhao 1, 2
Affiliation
|
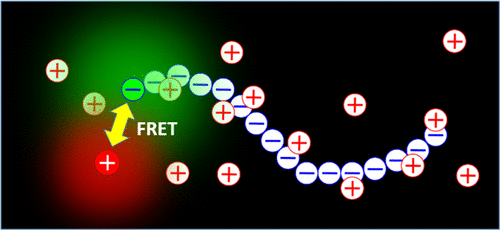
The dynamics of adsorption and desorption of counterions on a polyelectrolyte chain in aqueous solution is studied at a single molecular level. The investigation is through the process of fluorescence resonance energy transfer (FRET) between a fluorescence donor chemically attached to the chain end of a model polyanion (sodium polystyrene sulfonate, NaPSS) and a positively charged fluorescence acceptor (Atto 610), which also serves as the counterion probe in the solution. Dual-color fluorescence correlation spectroscopy (DC-FCS) is used and the results provide information on the rate constant for the counterion probe to adsorb onto and desorb from the charged main chain and multiple affecting factors. The desorption rate is constantly higher than the adsorption rate, making an overall balance towards desorption and leaving the main chain effectively charged. The adsorption rate increases linearly with the concentration of the counterion probes due to the reduced entropy penalty for adsorption, while the desorption rate remains unchanged. A lower desorption rate is found with the higher molecular weight sample and its adsorption rate shows a smaller change with the concentration, due to the less predominant entropy effect because of the higher surrounding counterion concentrations. Experiments with elevated salt concentrations (NaCl) demonstrate the adsorption competition between the sodium ion and the fluorescent probe and that the high salt level promotes more adsorption, making the chain less charged. Such a process induces a contraction of the chain from its original expanded conformation.
中文翻译:

聚电解质的抗衡离子结合动力学
在单个分子水平上研究了水溶液中聚电解质链上抗衡离子的吸附和解吸动力学。该研究是通过化学附着在模型聚阴离子(聚苯乙烯磺酸钠,NaPSS)链端的荧光供体与带正电的荧光受体(Atto 610)之间的荧光共振能量转移(FRET)过程进行的。溶液中的抗衡离子探针。使用了双色荧光相关光谱法(DC-FCS),结果提供了有关抗衡离子探针吸附到带电主链上或从带电主链上解吸的速率常数以及多种影响因素的信息。解吸速率始终高于吸附速率,总体上实现解吸平衡,并使主链有效充电。由于减少的吸附熵损失,吸附速率随抗衡离子探针的浓度线性增加,而解吸速率保持不变。较高分子量的样品发现较低的解吸速率,并且由于较高的周围抗衡离子浓度,主要的熵效应较小,因此其吸附速率随浓度变化较小。盐浓度(NaCl)升高的实验证明了钠离子与荧光探针之间的吸附竞争,并且高盐含量促进了更多的吸附,从而使链的电荷减少。这样的过程引起链从其原始的扩展构象收缩。由于减少的吸附熵损失,吸附速率随抗衡离子探针的浓度线性增加,而解吸速率保持不变。较高分子量的样品发现较低的解吸速率,并且由于较高的周围抗衡离子浓度,主要的熵效应较小,因此其吸附速率随浓度变化较小。盐浓度(NaCl)升高的实验证明了钠离子与荧光探针之间的吸附竞争,并且高盐含量促进了更多的吸附,从而使链的电荷减少。这样的过程引起链从其原始的扩展构象收缩。由于减少的吸附熵损失,吸附速率随抗衡离子探针的浓度线性增加,而解吸速率保持不变。较高分子量的样品发现较低的解吸速率,并且由于较高的周围抗衡离子浓度,主要的熵效应较小,因此其吸附速率随浓度变化较小。盐浓度(NaCl)升高的实验证明了钠离子与荧光探针之间的吸附竞争,并且高盐含量促进了更多的吸附,从而使链的电荷减少。这样的过程引起链从其原始的扩展构象收缩。
更新日期:2021-05-25
中文翻译:

聚电解质的抗衡离子结合动力学
在单个分子水平上研究了水溶液中聚电解质链上抗衡离子的吸附和解吸动力学。该研究是通过化学附着在模型聚阴离子(聚苯乙烯磺酸钠,NaPSS)链端的荧光供体与带正电的荧光受体(Atto 610)之间的荧光共振能量转移(FRET)过程进行的。溶液中的抗衡离子探针。使用了双色荧光相关光谱法(DC-FCS),结果提供了有关抗衡离子探针吸附到带电主链上或从带电主链上解吸的速率常数以及多种影响因素的信息。解吸速率始终高于吸附速率,总体上实现解吸平衡,并使主链有效充电。由于减少的吸附熵损失,吸附速率随抗衡离子探针的浓度线性增加,而解吸速率保持不变。较高分子量的样品发现较低的解吸速率,并且由于较高的周围抗衡离子浓度,主要的熵效应较小,因此其吸附速率随浓度变化较小。盐浓度(NaCl)升高的实验证明了钠离子与荧光探针之间的吸附竞争,并且高盐含量促进了更多的吸附,从而使链的电荷减少。这样的过程引起链从其原始的扩展构象收缩。由于减少的吸附熵损失,吸附速率随抗衡离子探针的浓度线性增加,而解吸速率保持不变。较高分子量的样品发现较低的解吸速率,并且由于较高的周围抗衡离子浓度,主要的熵效应较小,因此其吸附速率随浓度变化较小。盐浓度(NaCl)升高的实验证明了钠离子与荧光探针之间的吸附竞争,并且高盐含量促进了更多的吸附,从而使链的电荷减少。这样的过程引起链从其原始的扩展构象收缩。由于减少的吸附熵损失,吸附速率随抗衡离子探针的浓度线性增加,而解吸速率保持不变。较高分子量的样品发现较低的解吸速率,并且由于较高的周围抗衡离子浓度,主要的熵效应较小,因此其吸附速率随浓度变化较小。盐浓度(NaCl)升高的实验证明了钠离子与荧光探针之间的吸附竞争,并且高盐含量促进了更多的吸附,从而使链的电荷减少。这样的过程引起链从其原始的扩展构象收缩。































 京公网安备 11010802027423号
京公网安备 11010802027423号