当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Step Reductive Amination of 5-Hydroxymethylfurfural into 2,5-Bis(aminomethyl)furan over Raney Ni
ChemSusChem ( IF 7.5 ) Pub Date : 2021-04-28 , DOI: 10.1002/cssc.202100564 Zuojun Wei 1 , Yuran Cheng 2 , Kou Zhou 3 , Yue Zeng 3 , En Yao 2 , Qing Li 2 , Yingxin Liu 4 , Yong Sun 5
ChemSusChem ( IF 7.5 ) Pub Date : 2021-04-28 , DOI: 10.1002/cssc.202100564 Zuojun Wei 1 , Yuran Cheng 2 , Kou Zhou 3 , Yue Zeng 3 , En Yao 2 , Qing Li 2 , Yingxin Liu 4 , Yong Sun 5
Affiliation
|
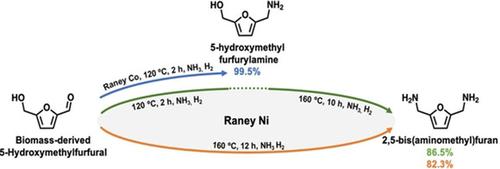
Simultaneous reductive amination of C=O and C−OH in 5-hydroxymethylfurfural (HMF) into C−NH2 in 2,5-bis(aminomethyl)furan (BAMF) is challenging. In this work, reductive amination of C=O in HMF was firstly studied, in which HMF can be converted into 5-hydroxymethyl furfurylamine (HMFA) with a 99.5 % yield over Raney Co catalyst. BAMF was then directly synthesized with 82.3 % yield from HMF over Raney Ni catalyst at 160 °C for 12 h. An even higher yield of 88.3 % could be obtained through a stepwise reductive amination process, in which the reaction started at 120 °C for the first 2 h over Raney Co mainly for amination of C=O and then continued at 160 °C for another 10 h over Raney Ni mainly for amination of C−OH. Under optimized reaction conditions, the catalyst could be reused four times without obvious loss in catalytic performance. XRD and XPS characterization of the reused catalyst indicated that the formation of Ni3N and the adsorption of alkyl amines could be the main reasons for the deactivation of the catalyst. Moreover, plausible reaction pathways were proposed to originate the detected by-products according to the kinetic profiles.
中文翻译:

雷尼镍上 5-羟甲基糠醛一步还原胺化成 2,5-双(氨甲基)呋喃
5-羟甲基糠醛 (HMF) 中的 C=O 和 C-OH 同时还原胺化成 C-NH 2在 2,5-双(氨基甲基)呋喃 (BAMF) 中具有挑战性。在这项工作中,首先研究了 HMF 中 C=O 的还原胺化,其中 HMF 可以在 Raney Co 催化剂上以 99.5% 的产率转化为 5-羟甲基糠胺 (HMFA)。然后在 160 °C 下,在 Raney Ni 催化剂上从 HMF 直接合成 BAMF,产率为 82.3%。通过逐步还原胺化过程可以获得甚至更高的 88.3% 的产率,其中在 Raney Co 上的前 2 小时反应在 120 °C 开始,主要用于胺化 C=O,然后在 160 °C 下继续进行另一个在 Raney Ni 上 10 小时,主要用于 C-OH 的胺化。在优化的反应条件下,催化剂可以重复使用4次,催化性能没有明显损失。再利用催化剂的 XRD 和 XPS 表征表明,Ni 的形成3 N 和烷基胺的吸附可能是催化剂失活的主要原因。此外,根据动力学曲线提出了可能的反应途径来产生检测到的副产物。
更新日期:2021-06-07
中文翻译:

雷尼镍上 5-羟甲基糠醛一步还原胺化成 2,5-双(氨甲基)呋喃
5-羟甲基糠醛 (HMF) 中的 C=O 和 C-OH 同时还原胺化成 C-NH 2在 2,5-双(氨基甲基)呋喃 (BAMF) 中具有挑战性。在这项工作中,首先研究了 HMF 中 C=O 的还原胺化,其中 HMF 可以在 Raney Co 催化剂上以 99.5% 的产率转化为 5-羟甲基糠胺 (HMFA)。然后在 160 °C 下,在 Raney Ni 催化剂上从 HMF 直接合成 BAMF,产率为 82.3%。通过逐步还原胺化过程可以获得甚至更高的 88.3% 的产率,其中在 Raney Co 上的前 2 小时反应在 120 °C 开始,主要用于胺化 C=O,然后在 160 °C 下继续进行另一个在 Raney Ni 上 10 小时,主要用于 C-OH 的胺化。在优化的反应条件下,催化剂可以重复使用4次,催化性能没有明显损失。再利用催化剂的 XRD 和 XPS 表征表明,Ni 的形成3 N 和烷基胺的吸附可能是催化剂失活的主要原因。此外,根据动力学曲线提出了可能的反应途径来产生检测到的副产物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号