Pharmacological Research ( IF 9.1 ) Pub Date : 2021-04-27 , DOI: 10.1016/j.phrs.2021.105640 Yunjie Wang 1 , Xin Guan 2 , Cheng-Long Gao 2 , Wenchen Ruan 2 , Shunyi Zhao 2 , Guoyin Kai 3 , Fei Li 2 , Tao Pang 4
|
Aim
Brain microvascular endothelial cells (BMVECs), as the important structure of blood-brain barrier (BBB), play a vital role in ischemic stroke. Pyroptosis of different cells in the brain may aggravate cerebral ischemic injury, and PGC-1α plays a major role in pyroptosis. However, it is not known whether BMVECs undergo pyroptosis after ischemic stroke and whether PGC-1α activator Medioresinol (MDN) we discovered may be useful against pyroptosis of endothelial cells and ischemic brain injury.
Methods
For in vitro experiments, the bEnd.3 cells and BMVECs under oxygen and glucose-deprivation (OGD) were treated with or without MDN, and the LDH release, tight junction protein degradation, GSDMD-NT membrane location and pyroptosis-associated proteins were evaluated. For in vivo experiments, mice underwent transient middle cerebral artery occlusion (tMCAO) for ischemia model, and the neuroprotective effects of MDN were measured by infarct volume, the permeability of BBB and pyroptosis of BMVECs. For mechanistic study, effects of MDN on the accumulation of phenylalanine, mitochondrial reactive oxygen species (mtROS) were tested by untargeted metabolomics and MitoSOX Red probe, respectively.
Results
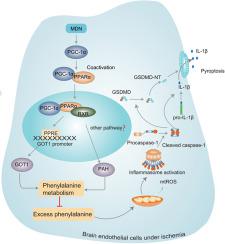
BMVECs underwent pyroptosis after ischemia. MDN dose-dependently activated PGC-1α, significantly reduced pyroptosis, mtROS and the expressions of pyroptosis-associated proteins (NLRP3, ASC, cleaved caspase-1, IL-1β, GSDMD-NT), and increased ZO-1 and Occludin protein expressions in BMVECs. In tMCAO mice, MDN remarkably reduced brain infarct volume and the permeability of BBB, inhibited pyroptosis of BMVECs, and promoted long-term neurobehavioral functional recovery. Mechanistically, MDN promoted the interaction of PGC-1α with PPARα to increase PPARα nuclear translocation and transcription activity, further increased the expression of GOT1 and PAH, resulting in enhanced phenylalanine metabolism to reduce the ischemia-caused phenylalanine accumulation and mtROS and further ameliorate pyroptosis of BMVECs.
Conclusion
In this study, we for the first time discovered that pyroptosis of BMVECs was involved in the pathogenesis of ischemic stroke and MDN as a novel PGC-1α activator could ameliorate the pyroptosis of endothelial cells and ischemic brain injury, which might attribute to reduction of mtROS through PPARα/GOT1 axis in BMVECs. Taken together, targeting endothelial pyroptosis by MDN may provide alternative therapeutics for brain ischemic stroke.
中文翻译:

Medioresinol 作为一种新型 PGC-1α 激活剂通过 PPARα-GOT1 轴预防缺血性中风内皮细胞焦亡
目的
脑微血管内皮细胞(BMVECs)作为血脑屏障(BBB)的重要结构,在缺血性脑卒中中发挥着至关重要的作用。脑内不同细胞的焦亡可能会加重脑缺血损伤,PGC-1α在细胞焦亡中起主要作用。然而,目前尚不清楚 BMVEC 是否会在缺血性中风后发生焦亡,以及我们发现的 PGC-1α 激活剂 Medioresinol (MDN) 是否可能有助于对抗内皮细胞焦亡和缺血性脑损伤。
方法
对于体外实验,缺氧和缺糖(OGD)下的 bEnd.3 细胞和 BMVEC 使用或不使用 MDN 进行处理,并评估了 LDH 释放、紧密连接蛋白降解、GSDMD-NT 膜定位和焦亡相关蛋白。在体内实验中,对小鼠进行短暂大脑中动脉闭塞(tMCAO)以建立缺血模型,并通过梗死体积、BBB通透性和BMVECs焦亡来测量MDN的神经保护作用。为了进行机理研究,分别通过非靶向代谢组学和 MitoSOX Red 探针测试了 MDN 对苯丙氨酸和线粒体活性氧 (mtROS) 积累的影响。
结果
BMVEC 缺血后发生细胞焦亡。 MDN 剂量依赖性激活 PGC-1α,显着减少焦亡、mtROS 和焦亡相关蛋白(NLRP3、ASC、cleaved caspase-1、IL-1β、GSDMD-NT)的表达,并增加 ZO-1 和 Occludin 蛋白表达在 BMVEC 中。在 tMCAO 小鼠中,MDN 显着减少脑梗死体积和 BBB 通透性,抑制 BMVEC 焦亡,促进长期神经行为功能恢复。机制上,MDN促进PGC-1α与PPARα相互作用,增加PPARα核转位和转录活性,进一步增加GOT1和PAH的表达,导致苯丙氨酸代谢增强,减少缺血引起的苯丙氨酸积累和mtROS,进一步改善细胞焦亡。 BMVEC。
结论
在本研究中,我们首次发现BMVECs的焦亡参与了缺血性中风的发病机制,而MDN作为一种新型PGC-1α激活剂可以改善内皮细胞的焦亡和缺血性脑损伤,这可能与mtROS的减少有关通过 BMVEC 中的 PPARα/GOT1 轴。总而言之,通过 MDN 靶向内皮细胞焦亡可能为脑缺血性中风提供替代疗法。































 京公网安备 11010802027423号
京公网安备 11010802027423号