当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterodimeric GW7604 Derivatives: Modification of the Pharmacological Profile by Additional Interactions at the Coactivator Binding Site
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-04-27 , DOI: 10.1021/acs.jmedchem.0c02230
Alexandra K Knox 1 , Christina Kalchschmid 1 , Daniela Schuster 1, 2 , Francesca Gaggia 1 , Ronald Gust 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-04-27 , DOI: 10.1021/acs.jmedchem.0c02230
Alexandra K Knox 1 , Christina Kalchschmid 1 , Daniela Schuster 1, 2 , Francesca Gaggia 1 , Ronald Gust 1
Affiliation
|
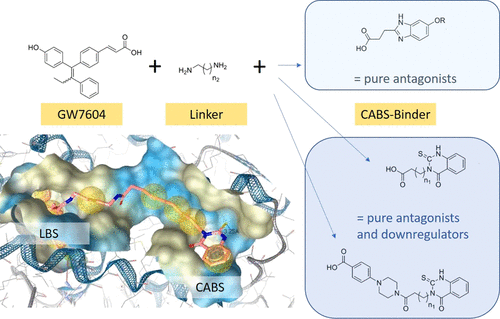
(E/Z)-3-(4-((E)-1-(4-Hydroxyphenyl)-2-phenylbut-1-enyl)phenyl)acrylic acid (GW7604) as a derivative of (Z)-4-hydroxytamoxifen (4-OHT) was linked by diaminoalkane spacers to molecules that are known binders to the coactivator binding site (benzimidazole or thioxo-quinazolinone scaffolds). With this modification, an optimization of the pharmacological profile was achieved. The most active thioxo-quinazolinone derivative 16 showed extraordinarily high affinity to the estrogen receptor (ER) β (RBA = 110%), inhibited effectively the coactivator recruitment (IC50 = 20.88 nM (ERα) and 28.34 nM (ERβ)), acted as a pure estradiol (E2) antagonist in a transactivation assay (IC50 = 18.5 nM (ERα) and 7.5 nM (ERβ)), and downregulated the ERα content in MCF-7 cells with an efficacy of 60% at 1 μM. The cytotoxicity was restricted to hormone-dependent MCF-7 (IC50 = 4.2 nM) and tamoxifen-resistant MCF-7TamR cells (IC50 = 476.6 nM). The compounds bearing a thioxo-quinazolinone moiety can therefore be assigned as pure E2-antagonistic selective ER degraders/downregulators. By contrast, the benzimidazole derivatives acted solely as pure antagonists without degradation of the ER.
中文翻译:

异二聚 GW7604 衍生物:通过辅助激活剂结合位点的额外相互作用改变药理学特征
(E / Z)-3-(4-((E)-1-(4-羟基苯基)-2-苯基丁-1-烯基)苯基)丙烯酸(GW7604)作为(Z)-4-羟基三苯氧胺的衍生物(4-OHT) 通过二氨基烷烃间隔基与作为共激活剂结合位点(苯并咪唑或硫代-喹唑啉酮支架)的已知结合剂的分子连接。通过这种修改,实现了药理学特征的优化。最活跃的硫代-喹唑啉酮衍生物16对雌激素受体 (ER) β 显示出极高的亲和力(RBA = 110%),有效抑制了辅激活因子的募集(IC 50 = 20.88 nM (ERα) 和 28.34 nM (ERβ)),起到了作用在反式激活试验中作为纯雌二醇 (E2) 拮抗剂 (IC 50= 18.5 nM (ERα) 和 7.5 nM (ERβ)),并下调 MCF-7 细胞中的 ERα 含量,在 1 μM 时的功效为 60%。细胞毒性仅限于激素依赖性 MCF-7 (IC 50 = 4.2 nM) 和他莫昔芬抗性 MCF-7TamR 细胞 (IC 50 = 476.6 nM)。因此,带有硫代-喹唑啉酮部分的化合物可以被指定为纯 E2 拮抗剂选择性 ER 降解剂/下调剂。相比之下,苯并咪唑衍生物仅作为纯拮抗剂而不会降解 ER。
更新日期:2021-05-13
中文翻译:

异二聚 GW7604 衍生物:通过辅助激活剂结合位点的额外相互作用改变药理学特征
(E / Z)-3-(4-((E)-1-(4-羟基苯基)-2-苯基丁-1-烯基)苯基)丙烯酸(GW7604)作为(Z)-4-羟基三苯氧胺的衍生物(4-OHT) 通过二氨基烷烃间隔基与作为共激活剂结合位点(苯并咪唑或硫代-喹唑啉酮支架)的已知结合剂的分子连接。通过这种修改,实现了药理学特征的优化。最活跃的硫代-喹唑啉酮衍生物16对雌激素受体 (ER) β 显示出极高的亲和力(RBA = 110%),有效抑制了辅激活因子的募集(IC 50 = 20.88 nM (ERα) 和 28.34 nM (ERβ)),起到了作用在反式激活试验中作为纯雌二醇 (E2) 拮抗剂 (IC 50= 18.5 nM (ERα) 和 7.5 nM (ERβ)),并下调 MCF-7 细胞中的 ERα 含量,在 1 μM 时的功效为 60%。细胞毒性仅限于激素依赖性 MCF-7 (IC 50 = 4.2 nM) 和他莫昔芬抗性 MCF-7TamR 细胞 (IC 50 = 476.6 nM)。因此,带有硫代-喹唑啉酮部分的化合物可以被指定为纯 E2 拮抗剂选择性 ER 降解剂/下调剂。相比之下,苯并咪唑衍生物仅作为纯拮抗剂而不会降解 ER。

































 京公网安备 11010802027423号
京公网安备 11010802027423号