Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
单原子 Cu/TiO2 光催化剂分解水:高效率的原子起源以及通过自旋选择增强的建议
JACS Au ( IF 8.5 ) Pub Date : 2021-04-26 , DOI: 10.1021/jacsau.1c00004
Cheng Cheng 1 , Wei-Hai Fang 1 , Run Long 1 , Oleg V Prezhdo 2
Affiliation
|
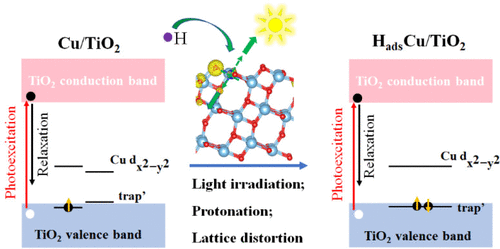
锐钛矿型 TiO 2因其丰度和化学稳定性而成为一种受到广泛研究的光催化材料。然而,它的光收集能力较弱,光催化效率较低。实验表明,TiO 2掺杂分散良好的Cu原子可以同时增强光吸收和光催化性能,形成单原子催化剂(Cu/TiO 2 ),可用于太阳能水分解等应用。通过从头开始非绝热分子动力学模拟,我们证明了 Cu/TiO 2在光照射之前由于通过浅陷阱和深陷阱的快速电子-空穴复合而处于不活跃状态。令人惊讶的是,浅陷阱比深陷阱对Cu/TiO 2性能更有害,因为它与自由载流子耦合得更好。光照射后,导致电子转移和Cu/TiO 2质子化,浅陷阱被消除,并且Cu原子周围的局部畸变稳定了Cu d轨道上的深陷阱状态,使其与自由电荷解耦并产生高光催化产氢活性。我们进一步证明,Cu/TiO 2的光催化性能可以通过自旋选择来增强,这可以通过光学位点间自旋转移或手性半导体涂层来实验实现。 H吸附和自旋选择都将载流子寿命提高了一个数量级。自旋选择机制不需要形成H物种,这需要同时存在电子和质子源,并且本质上不稳定,因为水分解涉及频繁的质子洗牌。 我们的结果合理化了原子水平上的实验观察,为单原子光催化的运行提供了机制见解,并证明自旋选择可用于开发先进且高效的太阳能转换系统。

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号