当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preferential Adsorption of Hydroxide Ions onto Partially Crystalline NiFe-Layered Double Hydroxides Leads to Efficient and Selective OER in Alkaline Seawater
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2021-04-27 , DOI: 10.1021/acsaem.1c00262 Qingqing Tu 1 , Wenwen Liu 1 , Meng Jiang 1 , Wenjuan Wang 1 , Qing Kang 1 , Pengcheng Wang 1 , Weijia Zhou 2 , Feimeng Zhou 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2021-04-27 , DOI: 10.1021/acsaem.1c00262 Qingqing Tu 1 , Wenwen Liu 1 , Meng Jiang 1 , Wenjuan Wang 1 , Qing Kang 1 , Pengcheng Wang 1 , Weijia Zhou 2 , Feimeng Zhou 1
Affiliation
|
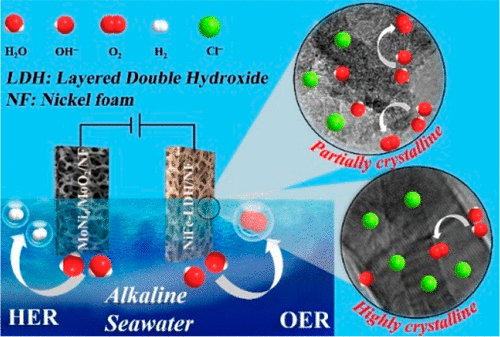
A variety of compounds, including Ni-, Fe-, and Co-containing layered double hydroxides (LDHs), have been explored as catalysts for the oxygen evolution reaction (OER). However, few can meet the industrially mandated overpotential of 0.30 V at 500 mA/cm2 and cell voltage of 1.60 V, let alone be applied to electrolysis of seawater. We synthesized a nickel foam (NF)-supported NiFe-LDH whose OER overpotential is only 0.257 V at 500 mA/cm2 in an alkaline saline solution and requires a cell voltage of 1.54 V for the same current density when coupled with a MoNi4/MoO2/NF cathode for electrolyzing alkalized seawater. The NiFe-LDH catalyst comprises numerous nanometer-sized crystalline facets surrounded by an amorphous phase, in contrast to its highly crystalline counterpart. X-ray photoelectron spectroscopy reveals that the boundaries separating crystalline facets and amorphous phase contain more Ni3+ than other areas. Anion chromatographic analysis indicates that OH– adsorbs preferentially over Cl– onto the sites of Ni3+ of both partially and highly crystalline NiFe-LDHs, whereas Cl– adsorbs more extensively onto the crystalline planes or facets. These adsorption behaviors and the resultant different catalytic activities at high current densities can be readily rationalized by the Pearson’s hard–soft acid–base principle. Because more boundaries exist in the partially crystalline NiFe-LDH, the partially crystalline NiFe-LDH catalyst is not only more catalytically efficient than its highly crystalline counterpart and other catalysts reported up to the present, but it is also stable in alkalized seawater and unaffected by Cl– adsorption.
中文翻译:

在部分结晶的NiFe层状双氢氧化物上优先吸附氢氧化物离子可在碱性海水中产生高效且选择性的OER
已经研究了多种化合物,包括含Ni,Fe和Co的层状双氢氧化物(LDHs)作为氧释放反应(OER)的催化剂。然而,很少有人能满足工业规定的500 mA / cm 2下0.30 V的过电势和1.60 V的电池电压,更不用说将其应用于海水电解了。我们合成了镍泡沫(NF)支撑的NiFe-LDH,其在碱性盐溶液中在500 mA / cm 2下的OER超电势仅为0.257 V,并且在与MoNi 4耦合时对于相同的电流密度需要1.54 V的电池电压/ MoO 2/ NF阴极,用于电解碱化海水。NiFe-LDH催化剂与高度结晶的对应物相比,包含被无定形相包围的许多纳米尺寸的结晶面。X射线光电子能谱表明,将晶面和非晶相分开的边界比其他区域包含更多的Ni 3+。阴离子色谱分析表明,OH -吸附优先于氯-到网站的Ni 3+两者部分地和高度结晶的NiFe-水滑石的,而氯-更加广泛地吸附到晶体平面或小平面上。这些吸附行为以及在高电流密度下产生的不同的催化活性可以很容易地通过皮尔逊的硬-软酸碱原理加以合理化。由于部分结晶的NiFe-LDH中存在更多的边界,因此部分结晶的NiFe-LDH催化剂不仅比其高度结晶的对等物和迄今为止报道的其他催化剂具有更高的催化效率,而且在碱化海水中也很稳定,并且不受Cl –吸附。
更新日期:2021-05-24
中文翻译:

在部分结晶的NiFe层状双氢氧化物上优先吸附氢氧化物离子可在碱性海水中产生高效且选择性的OER
已经研究了多种化合物,包括含Ni,Fe和Co的层状双氢氧化物(LDHs)作为氧释放反应(OER)的催化剂。然而,很少有人能满足工业规定的500 mA / cm 2下0.30 V的过电势和1.60 V的电池电压,更不用说将其应用于海水电解了。我们合成了镍泡沫(NF)支撑的NiFe-LDH,其在碱性盐溶液中在500 mA / cm 2下的OER超电势仅为0.257 V,并且在与MoNi 4耦合时对于相同的电流密度需要1.54 V的电池电压/ MoO 2/ NF阴极,用于电解碱化海水。NiFe-LDH催化剂与高度结晶的对应物相比,包含被无定形相包围的许多纳米尺寸的结晶面。X射线光电子能谱表明,将晶面和非晶相分开的边界比其他区域包含更多的Ni 3+。阴离子色谱分析表明,OH -吸附优先于氯-到网站的Ni 3+两者部分地和高度结晶的NiFe-水滑石的,而氯-更加广泛地吸附到晶体平面或小平面上。这些吸附行为以及在高电流密度下产生的不同的催化活性可以很容易地通过皮尔逊的硬-软酸碱原理加以合理化。由于部分结晶的NiFe-LDH中存在更多的边界,因此部分结晶的NiFe-LDH催化剂不仅比其高度结晶的对等物和迄今为止报道的其他催化剂具有更高的催化效率,而且在碱化海水中也很稳定,并且不受Cl –吸附。

































 京公网安备 11010802027423号
京公网安备 11010802027423号