Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study of catalytic hydrogenation and dehydrogenation of 2,3-dimethylindole for hydrogen storage application
RSC Advances ( IF 3.9 ) Pub Date : 2021-4-27 , DOI: 10.1039/d1ra01552d Yuan Dong 1 , Haoming Zhao 1 , Yinheng Zhao 1 , Ming Yang 1, 2 , Heshun Zhang 1 , Hansong Cheng 1
RSC Advances ( IF 3.9 ) Pub Date : 2021-4-27 , DOI: 10.1039/d1ra01552d Yuan Dong 1 , Haoming Zhao 1 , Yinheng Zhao 1 , Ming Yang 1, 2 , Heshun Zhang 1 , Hansong Cheng 1
Affiliation
|
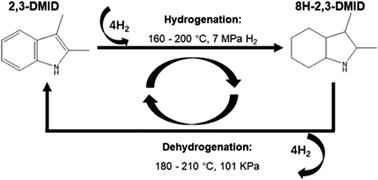
2,3-Dimethylindole (2,3-DMID), a candidate with a hydrogen storage capacity of 5.23 wt%, was studied as a new liquid organic hydrogen carrier (LOHC) in detail in this report. Hydrogenation of 2,3-DMID was conducted over 5 wt% Ru/Al2O3 by investigating the influences of temperature and hydrogen pressure. 100% of fully hydrogenated product, 8H-2,3-DMID can be achieved at 190 °C and 7 MPa in 4 h. Dehydrogenation of 8H-2,3-DMID was performed over 5 wt% Pd/Al2O3 at 180–210 °C and 101 kPa. It is found that dehydrogenation of 8H-2,3-DMID followed first order kinetics with an apparent activation energy of 39.6 kJ mol−1. The structures of intermediates produced in the 8H-2,3-DMID dehydrogenation process were analyzed by DFT calculations.
中文翻译:

2,3-二甲基吲哚催化加氢脱氢储氢应用研究
本报告详细研究了储氢能力为 5.23 wt% 的候选材料 2,3-二甲基吲哚 (2,3-DMID) 作为一种新型液态有机氢载体 (LOHC)。通过研究温度和氢气压力的影响,在5 wt% Ru/Al 2 O 3上进行2,3-DMID的氢化。在190℃、7MPa下4小时内可得到100%完全氢化的产物8H-2,3-DMID。 8H-2,3-DMID 的脱氢反应在 5 wt% Pd/Al 2 O 3中于 180–210 °C 和 101 kPa 下进行。研究发现8H-2,3-DMID的脱氢遵循一级动力学,表观活化能为39.6 kJ mol -1 。通过DFT计算分析了8H-2,3-DMID脱氢过程中产生的中间体的结构。
更新日期:2021-04-28
中文翻译:

2,3-二甲基吲哚催化加氢脱氢储氢应用研究
本报告详细研究了储氢能力为 5.23 wt% 的候选材料 2,3-二甲基吲哚 (2,3-DMID) 作为一种新型液态有机氢载体 (LOHC)。通过研究温度和氢气压力的影响,在5 wt% Ru/Al 2 O 3上进行2,3-DMID的氢化。在190℃、7MPa下4小时内可得到100%完全氢化的产物8H-2,3-DMID。 8H-2,3-DMID 的脱氢反应在 5 wt% Pd/Al 2 O 3中于 180–210 °C 和 101 kPa 下进行。研究发现8H-2,3-DMID的脱氢遵循一级动力学,表观活化能为39.6 kJ mol -1 。通过DFT计算分析了8H-2,3-DMID脱氢过程中产生的中间体的结构。































 京公网安备 11010802027423号
京公网安备 11010802027423号