当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring-contraction of hantzsch esters and their derivatives to pyrroles via electrochemical extrusion of ethyl acetate out of aromatic rings
Green Chemistry ( IF 9.3 ) Pub Date : 2021-4-8 , DOI: 10.1039/d1gc00487e Xu Liu 1, 2, 3, 4, 5 , Chang Liu 6, 7, 8, 9 , Xu Cheng 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2021-4-8 , DOI: 10.1039/d1gc00487e Xu Liu 1, 2, 3, 4, 5 , Chang Liu 6, 7, 8, 9 , Xu Cheng 1, 2, 3, 4, 5
Affiliation
|
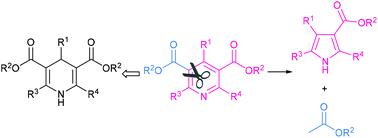
Electrochemical ring-contraction of HEs and theirs pyridine derivatives is developed to obtain polysubstituted pyrroles. This process provides an orthogonal utilization of Hantzsch esters for the well-documented application as side chain or hydrogen donors. The formal transformation shows an extrusion of ethyl acetate out of the pyridine ring in a single step. In addition to the novel transformation, we also discovered the Lewis acid's intermolecular control of regioselectivity during an intramolecular electrochemical process. The reaction provides a number of polysubstituted pyrroles that have never been accessed, including pharmaceutical intermediates and photoswitches. An unusual 4-electron continuous reduction drives the unprecedented anionic dearomatization/ring-contraction/rearomatization pathway.
中文翻译:

通过将乙酸乙酯从芳香环中电化学挤出,将汉茨酯及其衍生物环缩合为吡咯
开发了HEs及其吡啶衍生物的电化学环收缩法以获得多取代的吡咯。该方法提供了Hantzsch酯作为侧链或氢供体在正交文献中的正交应用。正式的转化显示了一步就将乙酸乙酯从吡啶环中挤出。除了新颖的转化,我们还发现了路易斯酸在分子内电化学过程中的区域选择性的分子间控制。该反应提供了许多从未得到的多取代的吡咯,包括药物中间体和光开关。不寻常的4电子连续还原反应驱动了前所未有的阴离子脱芳香化/环收缩/再芳香化途径。
更新日期:2021-04-27
中文翻译:

通过将乙酸乙酯从芳香环中电化学挤出,将汉茨酯及其衍生物环缩合为吡咯
开发了HEs及其吡啶衍生物的电化学环收缩法以获得多取代的吡咯。该方法提供了Hantzsch酯作为侧链或氢供体在正交文献中的正交应用。正式的转化显示了一步就将乙酸乙酯从吡啶环中挤出。除了新颖的转化,我们还发现了路易斯酸在分子内电化学过程中的区域选择性的分子间控制。该反应提供了许多从未得到的多取代的吡咯,包括药物中间体和光开关。不寻常的4电子连续还原反应驱动了前所未有的阴离子脱芳香化/环收缩/再芳香化途径。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号