当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidation of Active Sites for CH4 Catalytic Oxidation over Pd/CeO2 Via Tailoring Metal–Support Interactions
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-04-26 , DOI: 10.1021/acscatal.1c00839 Shiyuan Chen 1 , Songda Li 1 , Ruiyang You 1 , Ziyi Guo 2 , Fei Wang 1 , Guanxing Li 1 , Wentao Yuan 1 , Beien Zhu 3, 4 , Yi Gao 3, 4 , Ze Zhang 1 , Hangsheng Yang 1 , Yong Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-04-26 , DOI: 10.1021/acscatal.1c00839 Shiyuan Chen 1 , Songda Li 1 , Ruiyang You 1 , Ziyi Guo 2 , Fei Wang 1 , Guanxing Li 1 , Wentao Yuan 1 , Beien Zhu 3, 4 , Yi Gao 3, 4 , Ze Zhang 1 , Hangsheng Yang 1 , Yong Wang 1
Affiliation
|
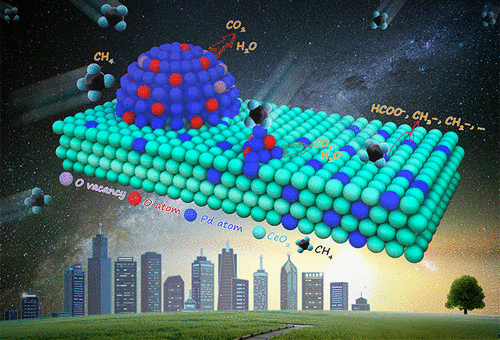
Pd/CeO2 has attracted great attention owing to its unique activity for methane catalytic oxidation; however, the active sites for CH4 catalytic oxidation still remain elusive, which affects the comprehensive understanding of the catalytic mechanism. In this work, the structures of PdOx nanoparticles (NPs) loaded on octahedrons, cubes, and rods of nanocrystal CeO2 supports were systematically studied by Cs-corrected HRTEM/STEM, XPS, and Raman spectroscopy. Our results indicate that the Pd species on CeO2 supports are morphology-dependent: PdO NPs (Pd2+) on octahedrons, PdOx (x = 1–2) clusters (1–2 nm) on cubes, and dispersed Pd4+ ions on the CeO2 rods. Additionally, the chemical states of Pd can be tuned in oxidizing/reducing atmospheres via interactions between Pd and CeO2. Detailed studies reveal that the Pd2+ species are the active centers for the catalytic oxidation of methane. The activity of Pd0 could be ascribed to Pd2+ produced through the gradual oxidation of Pd0 during the CH4 oxidation. Further, Pd4+ in the CeO2 lattice is inactive for CH4 oxidation. In situ Fourier transform infrared spectroscopy results suggest that the mechanism of CH4 oxidation reaction on PdOx/CeO2 follows the Mars–van Krevelen mechanism, and adsorbed CO can be produced in CH4 decomposition over Pd2+ in the absence of gas-phase oxygen. As revealed by density functional theory calculations, the incomplete coordination of Pd2+ ions and adjacent oxygen atoms has excellent activity in cracking the C–H bond of CH4, which leads to high methane oxidation ability.
中文翻译:

通过定制金属-载体相互作用阐明Pd / CeO 2上CH 4催化氧化的活性位点
Pd / CeO 2由于具有独特的甲烷催化氧化活性而备受关注。但是,CH 4催化氧化的活性位仍然难以捉摸,这影响了对催化机理的全面理解。在这项工作中,通过Cs校正的HRTEM / STEM,XPS和拉曼光谱系统地研究了负载在八面体,立方体和纳米晶CeO 2载体棒上的PdO x纳米颗粒(NP)的结构。我们的结果表明,CeO 2载体上的Pd种类取决于形态:八面体上的PdO NP(Pd 2+),立方体上的PdO x(x = 1-2)簇(1-2 nm)和分散的PdCeO 2棒上的4+离子。另外,可以通过Pd和CeO 2之间的相互作用在氧化/还原气氛中调节Pd的化学状态。详细的研究表明,Pd 2+物种是甲烷催化氧化的活性中心。Pd 0的活性可以归因于在CH 4氧化过程中Pd 0逐渐氧化产生的Pd 2+。另外,CeO 2晶格中的Pd 4+对于CH 4氧化是惰性的。原位傅里叶变换红外光谱结果表明CH 4的机理PdO x / CeO 2上的氧化反应遵循Mars–van Krevelen机理,在不存在气相氧的情况下,在Pd 2+上CH 4分解时可生成吸附的CO 。正如密度泛函理论计算所揭示的那样,Pd 2+离子与邻近的氧原子的不完全配位在裂解CH 4的C–H键方面具有出色的活性,从而具有很高的甲烷氧化能力。
更新日期:2021-05-07
中文翻译:

通过定制金属-载体相互作用阐明Pd / CeO 2上CH 4催化氧化的活性位点
Pd / CeO 2由于具有独特的甲烷催化氧化活性而备受关注。但是,CH 4催化氧化的活性位仍然难以捉摸,这影响了对催化机理的全面理解。在这项工作中,通过Cs校正的HRTEM / STEM,XPS和拉曼光谱系统地研究了负载在八面体,立方体和纳米晶CeO 2载体棒上的PdO x纳米颗粒(NP)的结构。我们的结果表明,CeO 2载体上的Pd种类取决于形态:八面体上的PdO NP(Pd 2+),立方体上的PdO x(x = 1-2)簇(1-2 nm)和分散的PdCeO 2棒上的4+离子。另外,可以通过Pd和CeO 2之间的相互作用在氧化/还原气氛中调节Pd的化学状态。详细的研究表明,Pd 2+物种是甲烷催化氧化的活性中心。Pd 0的活性可以归因于在CH 4氧化过程中Pd 0逐渐氧化产生的Pd 2+。另外,CeO 2晶格中的Pd 4+对于CH 4氧化是惰性的。原位傅里叶变换红外光谱结果表明CH 4的机理PdO x / CeO 2上的氧化反应遵循Mars–van Krevelen机理,在不存在气相氧的情况下,在Pd 2+上CH 4分解时可生成吸附的CO 。正如密度泛函理论计算所揭示的那样,Pd 2+离子与邻近的氧原子的不完全配位在裂解CH 4的C–H键方面具有出色的活性,从而具有很高的甲烷氧化能力。













































 京公网安备 11010802027423号
京公网安备 11010802027423号