European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-04-26 , DOI: 10.1016/j.ejmech.2021.113494 Maria Chiara Pismataro 1 , Tommaso Felicetti 1 , Chiara Bertagnin 2 , Maria Giulia Nizi 1 , Anna Bonomini 2 , Maria Letizia Barreca 1 , Violetta Cecchetti 1 , Dirk Jochmans 3 , Steven De Jonghe 3 , Johan Neyts 3 , Arianna Loregian 2 , Oriana Tabarrini 1 , Serena Massari 1
|
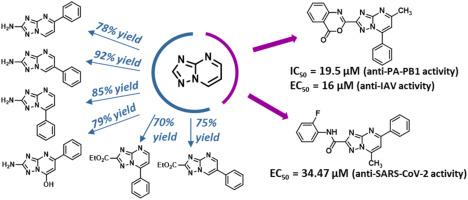
In the search for new anti-influenza virus (IV) compounds, we have identified the 1,2,4-triazolo[1,5-a]pyrimidine (TZP) as a very suitable scaffold to obtain compounds able to disrupt IV RNA-dependent RNA polymerase (RdRP) PA-PB1 subunits heterodimerization. In this work, in order to acquire further SAR insights for this class of compounds and identify more potent derivatives, we designed and synthesized additional series of analogues to investigate the role of the substituents around the TZP core. To this aim, we developed four facile and efficient one-step procedures for the synthesis of 5-phenyl-, 6-phenyl- and 7-phenyl-2-amino-[1,2,4]triazolo[1,5-a]pyrimidines, and 2-amino-5-phenyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-ol. Two analogues having the ethyl carboxylate moiety at the C-2 position of the TZP were also prepared in good yields. Then, the scaffolds herein synthesized and two previous scaffolds were functionalized and evaluated for their anti-IAV activity, leading to the identification of compound 22 that showed both anti-PA-PB1 (IC50 = 19.5 μM) and anti-IAV activity (EC50 = 16 μM) at non-toxic concentrations, thus resulting among the most active TZP derivatives reported to date by us. A selection of the synthesized compounds, along with a set of in-house available analogues, was also tested against SARS-CoV-2. The most promising compound 49 from this series displayed an EC50 value of 34.47 μM, highlighting the potential of the TPZ scaffold in the search for anti-CoV agents.
中文翻译:

1,2,4-三唑并[1,5- a ]嘧啶:高效的一步合成和功能化,作为流感聚合酶PA-PB1相互作用干扰物
在寻找新的抗流感病毒(IV)化合物时,我们已确定1,2,4-三唑并[1,5- a ]嘧啶(TZP)是非常合适的支架,以获得能够破坏IV RNA-依赖性RNA聚合酶(RdRP)PA-PB1亚基异二聚化。在这项工作中,为了获得有关此类化合物的更多SAR信息并确定更有效的衍生物,我们设计并合成了其他类似物系列,以研究TZP核周围取代基的作用。为此,我们开发了四个简便而有效的一步法合成5-苯基-,6-苯基-和7-苯基-2-氨基-[1,2,4]三唑[1,5- a ]嘧啶和2-氨基-5-苯基-[1,2,4]三唑[1,5- a] pyrimidin-7-ol。还以良好的产率制备了在TZP的C-2位具有羧酸乙酯部分的两个类似物。然后,将本文合成的支架和两个先前的支架进行功能化并评估其抗IAV活性,从而鉴定出同时显示抗PA-PB1(IC 50 = 19.5μM)和抗IAV活性(EC的化合物22)50 = 16μM),因此是我们迄今报告的活性最高的TZP衍生物之一。还针对SARS-CoV-2对一系列合成的化合物以及一组内部可用的类似物进行了测试。该系列中最有前途的化合物49表现出EC 50 值34.47μM,突出了TPZ支架在寻找抗CoV药物中的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号