Applied Surface Science ( IF 6.3 ) Pub Date : 2021-04-25 , DOI: 10.1016/j.apsusc.2021.149866
Jinchuan Zhang , Yingju Yang , Jing Liu , Bo Xiong
|
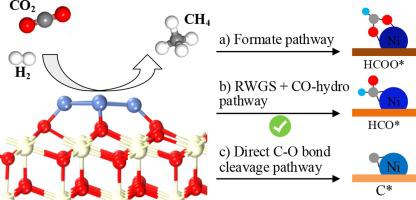
Ni/CeO2 is considered as a potentially efficient catalyst for CO2 methanation. Herein, all possible reaction pathways of CO2 methanation were investigated by the first-principles calculations based on density functional theory to uncover the intrinsic reaction mechanism over Ni/CeO2 catalyst. Theoretical analysis results indicate that the strong metal-support interaction between Ni nanoparticle and CeO2 support is closely associated with the transfer and accumulation of electrons towards the interface. Ni nanocluster on Ni/CeO2 catalyst is the most active adsorption and activation site of CO2 molecule. CO2 methanation over Ni/CeO2 catalyst is mainly governed by the RWGS + CO-hydro pathway, rather than the pathways of formate formation and direct C O bond cleavage. The main reaction channel of CO2 methanation is CO2* → HOCO* → CO* → HCO* → H2CO* → CH2* → CH3* → CH4*. H-assisted C
O bond cleavage. The main reaction channel of CO2 methanation is CO2* → HOCO* → CO* → HCO* → H2CO* → CH2* → CH3* → CH4*. H-assisted C O bond breakage of H2CO* plays a critical role in CO2 methanation, and is the rate-limiting step of RWGS + CO-hydro pathway. The optimal pathway and rate-limiting step of CO2 methanation are altered in the presence of CeO2. A complete reaction network is proposed for further understanding the molecular-level reduction mechanism of CO2 hydrogenation to methane on Ni/CeO2 catalyst.
O bond breakage of H2CO* plays a critical role in CO2 methanation, and is the rate-limiting step of RWGS + CO-hydro pathway. The optimal pathway and rate-limiting step of CO2 methanation are altered in the presence of CeO2. A complete reaction network is proposed for further understanding the molecular-level reduction mechanism of CO2 hydrogenation to methane on Ni/CeO2 catalyst.
中文翻译:

Ni / CeO 2催化剂上对CO 2加氢成甲烷的机理的理解
Ni / CeO 2被认为是用于CO 2甲烷化的潜在有效催化剂。在此,通过基于密度泛函理论的第一性原理计算,研究了所有可能的CO 2甲烷化反应途径,以揭示Ni / CeO 2催化剂的内在反应机理。理论分析结果表明,Ni纳米颗粒与CeO 2载体之间的强金属-载体相互作用与电子向界面的转移和积累密切相关。Ni / CeO 2催化剂上的Ni纳米簇是CO 2分子最活跃的吸附和活化位点。Ni / CeO上的CO 2甲烷化2催化剂主要由RWGS + CO-氢途径控制,而不是甲酸形成和直接C  O键裂解的途径。CO 2甲烷化的主要反应通道为CO 2 *→HOCO *→CO *→HCO *→H 2 CO *→CH 2 *→CH 3 *→CH 4 *。H 2 CO *
O键裂解的途径。CO 2甲烷化的主要反应通道为CO 2 *→HOCO *→CO *→HCO *→H 2 CO *→CH 2 *→CH 3 *→CH 4 *。H 2 CO * 的H辅助C O键断裂在CO 2甲烷化中起关键作用,并且是RWGS + CO-水途径的限速步骤。CeO 2的存在改变了CO 2甲烷化的最佳途径和限速步骤。为了进一步理解Ni / CeO 2催化剂上CO 2加氢成甲烷的分子级还原机理,提出了一个完整的反应网络。
的H辅助C O键断裂在CO 2甲烷化中起关键作用,并且是RWGS + CO-水途径的限速步骤。CeO 2的存在改变了CO 2甲烷化的最佳途径和限速步骤。为了进一步理解Ni / CeO 2催化剂上CO 2加氢成甲烷的分子级还原机理,提出了一个完整的反应网络。

































 京公网安备 11010802027423号
京公网安备 11010802027423号