Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2021-04-24 , DOI: 10.1016/j.jcis.2021.04.113 Bin Li , Jing Xue , Chao Han , Na Liu , Kaixuan Ma , Ruochen Zhang , Xianwen Wu , Lei Dai , Ling Wang , Zhangxing He
|
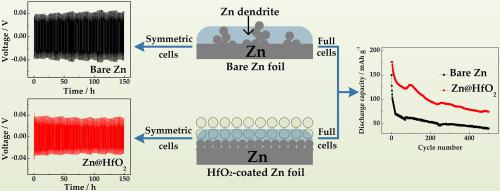
In aqueous zinc-ion batteries, metallic zinc is widely used as an anode because of its non-toxicity, environmental benignity, low cost, high abundance and theoretical capacity. However, growth of zinc dendrites, corrosion of zinc anode, passivation, and occurrence of side reactions during continuous charge-discharge cycling hinder development of zinc-ion batteries. In this study, a simple strategy involving application of a HfO2 coating was used to guide uniform deposition of Zn2+ to suppress formation of zinc dendrites. The HfO2-coated zinc anode improves electrochemical performance compared with bare Zn anode. Therefore, for zinc-zinc symmetric cells, zinc anode with HfO2 coating (48 mV) shows lower voltage hysteresis than that of bare Zn anode (63 mV) at a current density of 0.4 mA cm−2. Moreover, cell with HfO2 coating also shows good cycling performance in Zn-MnO2 full cells. At a constant current density of 1.0 A g−1, discharge capacity of bare Zn-MnO2 full cell is only 37.9 mAh g−1 after 500 cycles, while that of Zn@HfO2-MnO2 full cell is up to 78.3 mAh g−1. This good electrochemical performance may be the result of confinement effect and reduction of side reactions. Overall, a simple and beneficial strategy for future development of rechargeable aqueous zinc-ion batteries is provided.
中文翻译:

用于可充电含水锌离子电池的无氧化oxide涂层的无枝晶锌阳极
在水性锌离子电池中,金属锌由于其无毒,对环境无害,低成本,高丰度和理论容量而被广泛用作阳极。然而,锌枝晶的生长,锌阳极的腐蚀,钝化以及在连续的充放电循环过程中副反应的发生阻碍了锌离子电池的发展。在这项研究中,一种简单的策略涉及应用HfO 2涂层,以指导Zn 2+的均匀沉积,从而抑制锌枝晶的形成。与裸露的锌阳极相比,涂覆有HfO 2的锌阳极提高了电化学性能。因此,对于锌-锌对称电池,具有HfO 2的锌阳极在电流密度为0.4 mA cm -2的情况下,涂层(48 mV)表现出比裸露的锌阳极(63 mV)更低的电压滞后。此外,具有HfO 2涂层的电池在Zn-MnO 2充满电池中也显示出良好的循环性能。在1.0 A g -1的恒定电流密度下,裸露的Zn-MnO 2充满电池在500个循环后的放电容量仅为37.9 mAh g -1,而Zn @ HfO 2 -MnO 2充满电池的放电容量高达78.3 mAh g -1。这种良好的电化学性能可能是限制作用和副反应减少的结果。总体上,提供了一种简单且有益的策略,用于可再充电的水性锌离子电池的未来开发。

































 京公网安备 11010802027423号
京公网安备 11010802027423号