Tetrahedron ( IF 2.1 ) Pub Date : 2021-04-24 , DOI: 10.1016/j.tet.2021.132160 Brian J Levandowski 1 , Nile S Abularrage 1 , Ronald T Raines 1
|
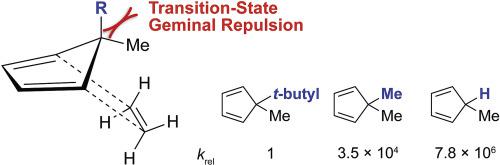
We have experimentally and computationally explored the sluggish Diels–Alder reactivities of the geminally substituted 5,5-dimethylcyclopentadiene and 5,5-dimethyl-2,3-diazacyclopentadiene (4,4-dimethyl-4H-pyrazole) scaffolds. We found that geminal dimethylation of 1,2,3,4-tetramethylcyclopentadiene to 1,2,3,4,5,5-hexamethylcyclopentadiene decreases the Diels–Alder reactivity towards maleimide by 954-fold. Quantum mechanical calculations revealed that the decreased Diels–Alder reactivities of gem-dimethyl substituted cyclopentadienes and 2,3-diazacyclopentadienes are not a consequence of unfavorable steric interactions between the diene and dienophile as reported previously, but a consequence of the increased repulsion within the gem-dimethyl group in the transition state. The findings have implications for the use of cyclopentadienes in “click” chemistry.
中文翻译:

双子排斥破坏双子取代的环戊二烯和 4H-吡唑的 Diels-Alder 反应
我们通过实验和计算探索了双取代的 5,5-二甲基环戊二烯和 5,5-二甲基-2,3-二氮杂环戊二烯(4,4-二甲基-4 H-吡唑)支架的缓慢 Diels-Alder 反应性。我们发现 1,2,3,4-四甲基环戊二烯到 1,2,3,4,5,5-六甲基环戊二烯的偕二甲基化使 Diels-Alder 对马来酰亚胺的反应性降低了 954 倍。量子力学计算表明,宝石-二甲基取代的环戊二烯和 2,3-二氮杂环戊二烯的 Diels-Alder 反应性降低并不是先前报道的二烯和亲二烯体之间不利的空间相互作用的结果,而是宝石内排斥力增加的结果-二甲基处于过渡态。这些发现对在“点击”化学中使用环戊二烯有影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号