当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly efficient ammonia synthesis at low temperature over a Ru–Co catalyst with dual atomically dispersed active centers
Chemical Science ( IF 7.6 ) Pub Date : 2021-4-7 , DOI: 10.1039/d1sc00304f Xuanbei Peng 1 , Han-Xuan Liu 2 , Yangyu Zhang 1 , Zheng-Qing Huang 2 , Linlin Yang 1 , Yafei Jiang 3 , Xiuyun Wang 1 , Lirong Zheng 4 , Chunran Chang 2 , Chak-Tong Au 1 , Lilong Jiang 1 , Jun Li 3, 5
Chemical Science ( IF 7.6 ) Pub Date : 2021-4-7 , DOI: 10.1039/d1sc00304f Xuanbei Peng 1 , Han-Xuan Liu 2 , Yangyu Zhang 1 , Zheng-Qing Huang 2 , Linlin Yang 1 , Yafei Jiang 3 , Xiuyun Wang 1 , Lirong Zheng 4 , Chunran Chang 2 , Chak-Tong Au 1 , Lilong Jiang 1 , Jun Li 3, 5
Affiliation
|
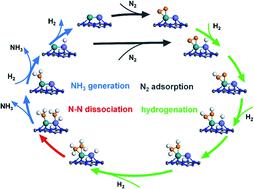
The desire for a carbon-free society and the continuously increasing demand for clean energy make it valuable to exploit green ammonia (NH3) synthesis that proceeds via the electrolysis driven Haber–Bosch (eHB) process. The key for successful operation is to develop advanced catalysts that can operate under mild conditions with efficacy. The main bottleneck of NH3 synthesis under mild conditions is the known scaling relation in which the feasibility of N2 dissociative adsorption of a catalyst is inversely related to that of the desorption of surface N-containing intermediate species, which leads to the dilemma that NH3 synthesis could not be catalyzed effectively under mild conditions. The present work offers a new strategy via introducing atomically dispersed Ru onto a single Co atom coordinated with pyrrolic N, which forms RuCo dual single-atom active sites. In this system the d-band centers of Ru and Co were both regulated to decouple the scaling relation. Detailed experimental and theoretical investigations demonstrate that the d-bands of Ru and Co both become narrow, and there is a significant overlapping of t2g and eg orbitals as well as the formation of a nearly uniform Co 3d ligand field, making the electronic structure of the Co atom resemble that of a “free-atom”. The “free-Co-atom” acts as a bridge to facilitate electron transfer from pyrrolic N to surface Ru single atoms, which enables the Ru atom to donate electrons to the antibonding π* orbitals of N2, thus resulting in promoted N2 adsorption and activation. Meanwhile, H2 adsorbs dissociatively on the Co center to form a hydride, which can transfer to the Ru site to cause the hydrogenation of the activated N2 to generate N2Hx (x = 1–4) intermediates. The narrow d-band centers of this RuCo catalyst facilitate desorption of surface *NH3 intermediates even at 50 °C. The cooperativity of the RuCo system decouples the sites for the activation of N2 from those for the desorption of *NH3 and *N2Hx intermediates, giving rise to a favorable pathway for efficient NH3 synthesis under mild conditions.
中文翻译:

具有双原子分散活性中心的 Ru-Co 催化剂在低温下高效合成氨
对无碳社会的渴望以及对清洁能源不断增长的需求使得开发通过电解驱动的哈伯-博世 (eHB) 工艺进行的绿色氨 (NH 3 ) 合成变得有价值。成功运行的关键是开发能够在温和条件下高效运行的先进催化剂。温和条件下NH 3合成的主要瓶颈是已知的结垢关系,其中催化剂N 2解离吸附的可行性与表面含氮中间体解吸的可行性成反比,这导致NH 3 的困境3的合成在温和条件下不能有效催化。目前的工作提供了一种新策略,通过将原子分散的Ru引入到与吡咯N配位的单个Co原子上,形成RuCo双单原子活性位点。在该系统中,Ru 和 Co 的 d 带中心均受到调节,以解耦标度关系。详细的实验和理论研究表明,Ru和Co的d带均变窄,t 2g和eg轨道显着重叠,并形成近乎均匀的Co 3d配体场,使得电子结构Co原子的结构类似于“自由原子”。 “游离Co原子”充当桥梁,促进电子从吡咯N转移到表面Ru单原子,这使得Ru原子能够将电子提供给N 2的反键π*轨道,从而促进N 2吸附和激活。同时,H 2解离吸附在Co中心上形成氢化物,氢化物可以转移到Ru位点,使活化的N 2氢化生成N 2 H x ( x = 1–4)中间体。即使在 50 °C 下,该 RuCo 催化剂的窄 d 带中心也有利于表面 *NH 3中间体的解吸。 RuCo系统的协同作用将N 2的活化位点与*NH 3和*N 2 H x中间体的解吸位点解耦,从而为在温和条件下高效合成NH 3提供了有利的途径。
更新日期:2021-04-23
中文翻译:

具有双原子分散活性中心的 Ru-Co 催化剂在低温下高效合成氨
对无碳社会的渴望以及对清洁能源不断增长的需求使得开发通过电解驱动的哈伯-博世 (eHB) 工艺进行的绿色氨 (NH 3 ) 合成变得有价值。成功运行的关键是开发能够在温和条件下高效运行的先进催化剂。温和条件下NH 3合成的主要瓶颈是已知的结垢关系,其中催化剂N 2解离吸附的可行性与表面含氮中间体解吸的可行性成反比,这导致NH 3 的困境3的合成在温和条件下不能有效催化。目前的工作提供了一种新策略,通过将原子分散的Ru引入到与吡咯N配位的单个Co原子上,形成RuCo双单原子活性位点。在该系统中,Ru 和 Co 的 d 带中心均受到调节,以解耦标度关系。详细的实验和理论研究表明,Ru和Co的d带均变窄,t 2g和eg轨道显着重叠,并形成近乎均匀的Co 3d配体场,使得电子结构Co原子的结构类似于“自由原子”。 “游离Co原子”充当桥梁,促进电子从吡咯N转移到表面Ru单原子,这使得Ru原子能够将电子提供给N 2的反键π*轨道,从而促进N 2吸附和激活。同时,H 2解离吸附在Co中心上形成氢化物,氢化物可以转移到Ru位点,使活化的N 2氢化生成N 2 H x ( x = 1–4)中间体。即使在 50 °C 下,该 RuCo 催化剂的窄 d 带中心也有利于表面 *NH 3中间体的解吸。 RuCo系统的协同作用将N 2的活化位点与*NH 3和*N 2 H x中间体的解吸位点解耦,从而为在温和条件下高效合成NH 3提供了有利的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号