Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-04-22 , DOI: 10.1016/j.bmc.2021.116168 Abdelfattah Hassan 1 , Mohamed Badr 2 , Heba A Hassan 3 , Dalia Abdelhamid 3 , Gamal El-Din A Abuo-Rahma 4
|
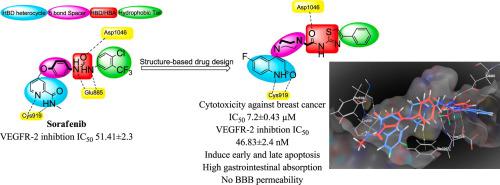
A new series of 2-(4-(2-oxo-1,2-dihydroquinolin-4-yl)piperazin-1-yl)-N-(4-phenylthiazol-2-yl)acetamide derivatives were synthesized and evaluated for anticancer activity. All target compounds showed anticancer activity higher than that of their 2-oxo-4-piperazinyl-1,2-dihydroquinolin-2(1H)-one precursors. Multidose testing of target compounds was performed against breast cancer T-47D cell line. Five compounds showed higher cytotoxic activity than Staurosporine. The dihalogenated derivative showed the best cytotoxic activity with IC50 2.73 ± 0.16 µM. In addition, the VEGFR-2 inhibitory activity of all synthetic compounds was evaluated. Two compounds of 6-fluoro-4-(piperazin-1-yl)quinolin-2(1H)-ones showed inhibitory activity comparable to sorafenib with IC50 46.83 ± 2.4, 51.09 ± 2.6 and 51.41 ± 2.3 nM, respectively. The cell cycle analysis of two compounds namely, 2-(4-(6-fluoro-2-oxo-1,2-dihydroquinolin-4-yl)piperazin-1-yl)-N-(4-phenylthiazol-2-yl)acetamide and N-(4-(4-chlorophenyl)thiazol-2-yl)-2-(4-(2-oxo-1-phenyl-1,2-dihydroquinolin-4-yl)piperazin-1-yl)acetamide revealed that the arrest of cell cycle occurred at S phase. In apoptosis assay, the same two compounds were able to induce significant levels of early and late apoptosis. In a similar manner to Sorafenib, docking of target compounds with VEGFR-2 protein 4ASD showed HB with Cys919 in hinge region of enzyme and HB with both Glu885 and Asp1046 in gate area. Using SwissADME, all target compounds were predicted to be highly absorbed from gastrointestinal tract with no BBB permeability. It is clear that the two compounds are promising antiproliferative candidates that require further optimization.
中文翻译:

通过 VEGFR-2-TK 抑制具有抗增殖活性的新型 4-(piperazin-1-yl)quinolin-2(1H)-one 含噻唑
合成了一系列新的 2-(4-(2-oxo-1,2-dihydroquinolin-4-yl)piperazin-1-yl) -N- (4-phenylthiazol-2-yl) 乙酰胺衍生物并评价其抗癌作用活动。所有目标化合物的抗癌活性均高于其 2-oxo-4-piperazinyl-1,2-dihydroquinolin-2(1 H )-one 前体。针对乳腺癌 T-47D 细胞系进行了目标化合物的多剂量测试。五种化合物显示出比星形孢菌素更高的细胞毒活性。二卤代衍生物显示出最佳的细胞毒活性,IC 50 为2.73 ± 0.16 µM。此外,还评估了所有合成化合物的 VEGFR-2 抑制活性。6-fluoro-4-(piperazin-1-yl)quinolin-2(1 H )的两种化合物)-ones 显示出与索拉非尼相当的抑制活性,IC 50 分别为46.83 ± 2.4、51.09 ± 2.6 和 51.41 ± 2.3 nM。2-(4-(6-fluoro-2-oxo-1,2-dihydroquinolin-4-yl)piperazin-1-yl) -N- (4-phenylthiazol-2-yl ) 两种化合物的细胞周期分析)乙酰胺和N-(4-(4-chlorophenyl)thiazol-2-yl)-2-(4-(2-oxo-1-phenyl-1,2-dihydroquinolin-4-yl)piperazin-1-yl)acetamide 表明细胞周期停滞发生在 S 期。在细胞凋亡试验中,相同的两种化合物能够诱导显着水平的早期和晚期细胞凋亡。以与索拉非尼类似的方式,目标化合物与 VEGFR-2 蛋白 4ASD 的对接显示 HB 与 Cys919 位于酶的铰链区,HB 与 Glu885 和 Asp1046 位于门区。使用 SwissADME,预测所有目标化合物都可从胃肠道高度吸收,没有 BBB 渗透性。很明显,这两种化合物是有前途的抗增殖候选物,需要进一步优化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号