当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis of D-Phenylalanine from L-Phenylalanine via a Tri-Enzymatic Cascade Pathway
ChemCatChem ( IF 3.8 ) Pub Date : 2021-04-22 , DOI: 10.1002/cctc.202100237 Lu Cui 1 , Zhang Sheng 2 , Song Wei 3 , Chen Xiulai 3 , Liu Jia 3 , Liu Liming 3 , Wu Jing 4
ChemCatChem ( IF 3.8 ) Pub Date : 2021-04-22 , DOI: 10.1002/cctc.202100237 Lu Cui 1 , Zhang Sheng 2 , Song Wei 3 , Chen Xiulai 3 , Liu Jia 3 , Liu Liming 3 , Wu Jing 4
Affiliation
|
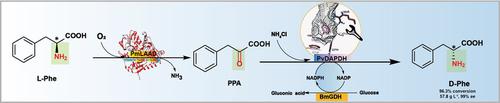
D-phenylalanine is an important intermediate in food and pharmaceutical industries. Here, to enable efficient D-phenylalanine biosynthesis from L-phenylalanine, a tri-enzymatic cascade was designed and reconstructed in vivo. The activity of Proteus vulgaris meso-diaminopimelate dehydrogenase (PvDAPDH) toward phenyl pyruvic acid was identified as the limiting step. To overcome, the tension in the phenyl pyruvic acid side-chain, PvDAPDH was engineered, generating PvDAPDHW121A/R181S/H227I, whose catalytic activity of 6.86 U mg−1 represented an 85-fold increase over PvDAPDH. Introduction of PvDAPDHW121A/R181S/H227I, P. mirabilis L-amino acid deaminase, and Bacillus megaterium glucose dehydrogenase in E. coli enabled the production of 57.8 g L−1 D-phenylalanine in 30 h, the highest titer to date using 60 g L−1 L-phenylalanine as starting substrate, which meant a 96.3 % conversion rate and >99 % enantioselectivity on a 3-L scale. The proposed tri-enzymatic cascade provides a novel potential bio-based approach for industrial production of D-phenylalanine from cheap amino acids.
中文翻译:

通过三酶级联途径从 L-苯丙氨酸有效合成 D-苯丙氨酸
D-苯丙氨酸是食品和制药行业的重要中间体。在这里,为了能够从 L-苯丙氨酸有效地生物合成 D-苯丙氨酸,在体内设计和重建了三酶级联反应。普通变形杆菌内消旋二氨基庚二酸脱氢酶 (PvDAPDH) 对苯基丙酮酸的活性被确定为限制步骤。为了克服这一问题,对苯基丙酮酸侧链 PvDAPDH 中的张力进行了改造,生成了 PvDAPDH W121A/R181S/H227I,其催化活性为 6.86 U mg -1 ,比 PvDAPDH 提高了 85 倍。引入 PvDAPDH W121A/R181S/H227I、奇异假单胞菌L-氨基酸脱氨酶和巨大芽孢杆菌的葡萄糖脱氢酶在大肠杆菌中能够生产57.8克升-1 d苯丙氨酸,以30小时,使用含有60克L-最高滴度迄今为止-1 L-苯丙氨酸作为起始衬底,这意味着一个96.3%的转化率和在 3 L 规模上具有 >99% 的对映选择性。所提出的三酶级联为从廉价氨基酸工业生产 D-苯丙氨酸提供了一种新的潜在的基于生物的方法。
更新日期:2021-04-22
中文翻译:

通过三酶级联途径从 L-苯丙氨酸有效合成 D-苯丙氨酸
D-苯丙氨酸是食品和制药行业的重要中间体。在这里,为了能够从 L-苯丙氨酸有效地生物合成 D-苯丙氨酸,在体内设计和重建了三酶级联反应。普通变形杆菌内消旋二氨基庚二酸脱氢酶 (PvDAPDH) 对苯基丙酮酸的活性被确定为限制步骤。为了克服这一问题,对苯基丙酮酸侧链 PvDAPDH 中的张力进行了改造,生成了 PvDAPDH W121A/R181S/H227I,其催化活性为 6.86 U mg -1 ,比 PvDAPDH 提高了 85 倍。引入 PvDAPDH W121A/R181S/H227I、奇异假单胞菌L-氨基酸脱氨酶和巨大芽孢杆菌的葡萄糖脱氢酶在大肠杆菌中能够生产57.8克升-1 d苯丙氨酸,以30小时,使用含有60克L-最高滴度迄今为止-1 L-苯丙氨酸作为起始衬底,这意味着一个96.3%的转化率和在 3 L 规模上具有 >99% 的对映选择性。所提出的三酶级联为从廉价氨基酸工业生产 D-苯丙氨酸提供了一种新的潜在的基于生物的方法。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号