当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bifunctional Primary Amino-thiourea Asymmetric Catalysis: The Imine-Iminium Ion Mechanism in the Michael Addition of Nitromethane to Enone
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2021-04-22 , DOI: 10.1002/ajoc.202100160 Virginia Rufino 1 , Josefredo Rodriguez Pliego 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2021-04-22 , DOI: 10.1002/ajoc.202100160 Virginia Rufino 1 , Josefredo Rodriguez Pliego 2
Affiliation
|
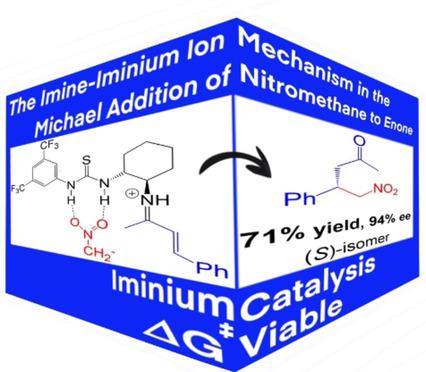
Primary-amine thioureas are preeminent organocatalysts used in challenging asymmetric Michael-type reactions, such as in the addition of nitromethane to enones. However, the complete reaction mechanism of this reaction has not yet been fully elucidated. Considering that the primary-amine group could act via both base and iminium ion catalysis, both mechanisms were investigated in this work using theoretical methods. The calculations have indicated that the base catalysis is kinetically unfeasible, with very high overall ΔG≠. An imine-iminium ion catalysis is a viable route. This mechanism requires the participation of an acid as a cocatalyst (additive) in several steps, including the formation of the imine. The rate-determining step corresponds to the proton transfer from the nitromethane to the imine, forming the nitronate-iminium ion-pair intermediate. This complex reacts via nucleophilic attack of the nitronate ion to the electrophilic carbon of the iminium ion, forming a new C−C bond that determines the enantioselectivity of the reaction.
中文翻译:

双功能伯氨基-硫脲不对称催化:硝基甲烷与烯酮迈克尔加成反应中的亚胺-亚胺离子机理
伯胺硫脲是卓越的有机催化剂,用于挑战不对称迈克尔型反应,例如硝基甲烷与烯酮的加成。然而,该反应的完整反应机理尚未完全阐明。考虑到伯胺基团可以通过碱和亚胺离子催化作用,在这项工作中使用理论方法研究了这两种机制。计算表明碱催化在动力学上是不可行的,总体ΔG ≠. 亚胺-亚胺离子催化是一种可行的途径。这种机制需要酸作为助催化剂(添加剂)参与几个步骤,包括亚胺的形成。速率决定步骤对应于从硝基甲烷到亚胺的质子转移,形成硝基-亚胺离子对中间体。该复合物通过硝基离子对亚胺离子的亲电碳的亲核攻击发生反应,形成一个新的 C-C 键,决定了反应的对映选择性。
更新日期:2021-06-10
中文翻译:

双功能伯氨基-硫脲不对称催化:硝基甲烷与烯酮迈克尔加成反应中的亚胺-亚胺离子机理
伯胺硫脲是卓越的有机催化剂,用于挑战不对称迈克尔型反应,例如硝基甲烷与烯酮的加成。然而,该反应的完整反应机理尚未完全阐明。考虑到伯胺基团可以通过碱和亚胺离子催化作用,在这项工作中使用理论方法研究了这两种机制。计算表明碱催化在动力学上是不可行的,总体ΔG ≠. 亚胺-亚胺离子催化是一种可行的途径。这种机制需要酸作为助催化剂(添加剂)参与几个步骤,包括亚胺的形成。速率决定步骤对应于从硝基甲烷到亚胺的质子转移,形成硝基-亚胺离子对中间体。该复合物通过硝基离子对亚胺离子的亲电碳的亲核攻击发生反应,形成一个新的 C-C 键,决定了反应的对映选择性。













































 京公网安备 11010802027423号
京公网安备 11010802027423号